Theme: Biosimilars- Uncovering An innovation In Motion
Asian Biosimilars 2017
8th Asian Biologics and Biosimilars Congress 2017 Organizing Committee invites you to attend the largest assemblage of biologics and biosimilars researchers from around the globe during August 10-12, 2017 at Beijing, China.
Asian Biosimilars Congress is a global annual event. This Biosimilars Congress 2017 brings together scientists, researchers, business development managers, CEOs, directors, IP Attorneys, Regulatory Officials and CROs from around the world. The passage of biosimilars through a decade at Asia finds much requirement for discussion also focusing the latest developments in the field of biologics and biosimilars. A comprehensive approach for the discussion has been taken up as listed below under several tracks and sub-tracks below:
Track 1: Latest Biosimilars in Asian Scenario
Biosimilars are surmounting pharmaceutical business division from latest three decades and arrangements appear to extend logically. Progresses in the biotechnology lead to improvement and disclosure of new biological products (biosimilars) to treat different life-threatening diseases. Biosimilars are biological drugs that are conveyed after expiry of the patent of affirmed innovator. This track attempts to highlight the various prospects of biosimilars in Asian market, Market strategy, Market Analysis, etc.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
8th Annual Biosimilars Asia, May 15-18, 2017, Shanghai, China; World Biosimilar Congress, November 14-15, 2016, Basel, Switzerland; Biosimilars & Biobetters Congress, April 2017, London, UK; 3rd Biologics & Biosimilars Congress, March 6-7, 2017, Berlin, Germany; Biosimilars Europe Congress 2017, November 21-22, 2017, London, UK; 8th Global Pharmacovigilance & Drug Safety Summit, July 10-11, 2017 Jakarta, Indonesia; 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia; 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea; 11th International Conference on Pharmacoepidemiology and Clinical Research, July 06-08, 2017 Kuala Lumpur, Malaysia; 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia
BioSim-Asociación Española de Biosimilares , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association , IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 2: Biosimilars- Hatchwaxman Act & BPCI Act
Hatch-Waxman act is the amendment to Federal, Food, Drug and Cosmetics act which established the modern system of approval of generics through Abbreviated New Drug Applications (ANDAs). The Patient Protection and Affordable Care Act (PPAC Act) was signed into law in March 2010 notwithstanding the alterations in the Public Health Service Act (PHS Act) to create an abbreviated approval pathway for biosimilars and follow on biologics. These new statutory provisions are often referred as the Biologics Price Competition and Innovation Act of 2009 (BPCI Act). This track concentrates upon such lawful ties and the aspects of the BPCI Act that pertain to the biosimilars and biologics. This session on legal issues shall be very beneficial to research scientists from both academic backgrounds and also those from industry R&D. The Asia Pacific Biosimilars that includes the Japan biosimilars, China biosimilars, Australian biosimilars, Indian biosimilars and Korean biosimilars are expected to take control over the biosimilar market around the globe by 2017.
Related Conferences: Biosimilar Symposiums | Biologics Conferences:
8th Annual Biosimilars Asia, May 15-18, 2017, Shanghai, China; World Biosimilar Congress, November 14-15, 2016, Basel, Switzerland; Biosimilars & Biobetters Congress, April 2017, London, UK; 3rd Biologics & Biosimilars Congress, March 6-7, 2017, Berlin, Germany; Biosimilars Europe Congress 2017, November 21-22, 2017, London, UK; 8th Global Pharmacovigilance & Drug Safety Summit, July 10-11, 2017 Jakarta, Indonesia; 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia; 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea; 11th International Conference on Pharmacoepidemiology and Clinical Research, July 06-08, 2017 Kuala Lumpur, Malaysia; 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia
BioSim-Asociación Española de Biosimilares , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association , IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 3: Challenges in Developing Biosimilars
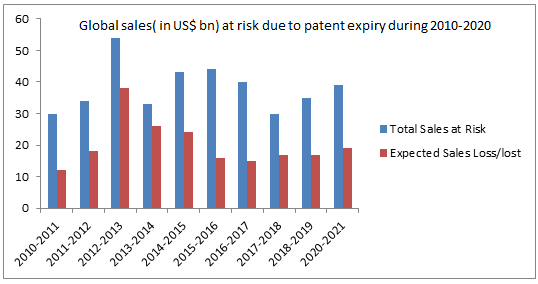
With an expected $67 billion worth of patents on biological products terminating before 2020 and governments pressured to reduce rapidly rising health care costs, biosimilars represent a major opportunity for the pharmaceutical industry. Biosimilars are highly similar to their licensed reference product not withstanding minor contrasts as excipients in the formulation; additionally there are no remarkable differences between the biologicals and the reference product in terms of safety, purity, and potency. However, there are certain challenges in way of its advancement and receiving a green flag for propelling into the market. Over the past ten years, regulatory authorities worldwide have been focusing on developing guidelines for biosimilars. However, until a global development strategy is adopted, regulatory, therapeutic and legal challenges remain.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
8th Annual Biosimilars Asia, May 15-18, 2017, Shanghai, China; World Biosimilar Congress, November 14-15, 2016, Basel, Switzerland; Biosimilars & Biobetters Congress, April 2017, London, UK; 3rd Biologics & Biosimilars Congress, March 6-7, 2017, Berlin, Germany; Biosimilars Europe Congress 2017, November 21-22, 2017, London, UK; 8th Global Pharmacovigilance & Drug Safety Summit, July 10-11, 2017 Jakarta, Indonesia; 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia; 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea; 11th International Conference on Pharmacoepidemiology and Clinical Research, July 06-08, 2017 Kuala Lumpur, Malaysia; 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia
BioSim-Asociación Española de Biosimilares , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association , IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 4: Biosimilars Research Pipeline
Biosimilars is a biologic therapeutic product which is duplicate of an original product that is manufactured by a different company. Biosimilars are officially approved versions of original biosimilar innovator products, and can be manufactured when the original product's patent expires. This session would be very helpful for the biosimilar business specialists to update themselves on the latest research updates from around the world. This session also finds place for all the biosimilar exhibitions associated with the field of biosimilars and biologics.
Related Conferences:
8th Annual Biosimilars Asia, May 15-18, 2017, Shanghai, China; World Biosimilar Congress, November 14-15, 2016, Basel, Switzerland; Biosimilars & Biobetters Congress, April 2017, London, UK; 3rd Biologics & Biosimilars Congress, March 6-7, 2017, Berlin, Germany; Biosimilars Europe Congress 2017, November 21-22, 2017, London, UK; 8th Global Pharmacovigilance & Drug Safety Summit, July 10-11, 2017 Jakarta, Indonesia; 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia; 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea; 11th International Conference on Pharmacoepidemiology and Clinical Research, July 06-08, 2017 Kuala Lumpur, Malaysia; 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia
BioSim-Asociación Española de Biosimilares , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association , IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 5: Current Developments in the Field of Biosimilars
Biologic therapeutic products created through rDNA technology as recombinant protein-based mediciness that have been in clinical use since the early 1980s as original biopharmaceuticals have greatly contributed to the therapy of severe metabolic and degenerative diseases. The recent expiration of the data protection or patents for most of them created opportunities for the development of copy versions of original biopharmaceuticals with similar biologic activity (termed biosimilars). Production of these new products is expected to meet worldwide demand, promote market competition, maintain the incentives for innovation, and sustain the healthcare systems.
Related Conferences: Biologics Conferences | Biologics Meetings:
8th Annual Biosimilars Asia, May 15-18, 2017, Shanghai, China; World Biosimilar Congress, November 14-15, 2016, Basel, Switzerland; Biosimilars & Biobetters Congress, April 2017, London, UK; 3rd Biologics & Biosimilars Congress, March 6-7, 2017, Berlin, Germany; Biosimilars Europe Congress 2017, November 21-22, 2017, London, UK; 8th Global Pharmacovigilance & Drug Safety Summit, July 10-11, 2017 Jakarta, Indonesia; 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia; 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea; 11th International Conference on Pharmacoepidemiology and Clinical Research, July 06-08, 2017 Kuala Lumpur, Malaysia; 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia
BioSim-Asociación Española de Biosimilares , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association , IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 6: Pharmacovigilance of Biosimilars
This session of Asian Biosimilars 2017 investigates the future and FDA activities that have already been announced to include enhanced tracking and follow-up of post market issues, planned improvements in AERS, and pilots of new post market drug-monitoring strategies. Current challenges in pharmacovigilance, Adverse drug responses with pharmaceutical products, Biosimilar rules for Pharmacovigilance operation and pharmacoepidemiology are the points that shall be laid emphasis in this session.
Related Biosimilar Conferences | Biologics Meetings:
8th Annual Biosimilars Asia, May 15-18, 2017, Shanghai, China; World Biosimilar Congress, November 14-15, 2016, Basel, Switzerland; Biosimilars & Biobetters Congress, April 2017, London, UK; 3rd Biologics & Biosimilars Congress, March 6-7, 2017, Berlin, Germany; Biosimilars Europe Congress 2017, November 21-22, 2017, London, UK; 8th Global Pharmacovigilance & Drug Safety Summit, July 10-11, 2017 Jakarta, Indonesia; 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia; 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea; 11th International Conference on Pharmacoepidemiology and Clinical Research, July 06-08, 2017 Kuala Lumpur, Malaysia; 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia
BioSim-Asociación Española de Biosimilares , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association , IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 7: Emerging Trends in Biosimilars
As patents of the initially presented biological therapeutics in oncology have started to lapse, competing pharmaceutical companies are allowed to produce and market the same protein as the originator agent. This follows the pattern of the development of generics. However, biosimilars are basically dissimilar from generics. Especially in the field of oncology, the introduction of monoclonal antibodies has resulted in spectacular therapeutic advances by increasing the cure rate of early cancers and prolonging survival. Similar advances have occurred in rheumatology, haematology, neurology and different fields. The effectiveness of granulocyte colony stimulating factor (G-CSF; filgrastim) biosimilars has been assessed in terms of engraftment following stem-cell transplantation. Time to engraftment was compared following treatment with the originator, Neupogen (Amgen), and with biosimilars in a retrospective, single-institution study.
Related Conferences: Biologics Conferences | Biologics Meetings:
8th Annual Biosimilars Asia, May 15-18, 2017, Shanghai, China; World Biosimilar Congress, November 14-15, 2016, Basel, Switzerland; Biosimilars & Biobetters Congress, April 2017, London, UK; 3rd Biologics & Biosimilars Congress, March 6-7, 2017, Berlin, Germany; Biosimilars Europe Congress 2017, November 21-22, 2017, London, UK; 8th Global Pharmacovigilance & Drug Safety Summit, July 10-11, 2017 Jakarta, Indonesia; 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia; 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea; 11th International Conference on Pharmacoepidemiology and Clinical Research, July 06-08, 2017 Kuala Lumpur, Malaysia; 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia
BioSim-Asociación Española de Biosimilares , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association , IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 8: Biosimilars Market- Challenges & Prospects
This track is concentrated towards the distinctive evaluations and projections with respect to the situation of Biosimilars market and follow on Biologics. The present status and future scenario of biosimilars market are best to be discussed during this session. Market inquires about from the primary launching of biosimilar to the most current one till date prospects for a radical change in the pharmaceutical market. Market researchers, market analysts, industrialists would be the apt participants for this session at Asian Biosimilars 2017.
Related Conferences: Biosimilar Conferences | Biologics Workshops:
8th Annual Biosimilars Asia, May 15-18, 2017, Shanghai, China; World Biosimilar Congress, November 14-15, 2016, Basel, Switzerland; Biosimilars & Biobetters Congress, April 2017, London, UK; 3rd Biologics & Biosimilars Congress, March 6-7, 2017, Berlin, Germany; Biosimilars Europe Congress 2017, November 21-22, 2017, London, UK; 8th Global Pharmacovigilance & Drug Safety Summit, July 10-11, 2017 Jakarta, Indonesia; 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia; 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea; 11th International Conference on Pharmacoepidemiology and Clinical Research, July 06-08, 2017 Kuala Lumpur, Malaysia; 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia
BioSim-Asociación Española de Biosimilares , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association , IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 9: Cost Analysis of Biosimilars
More than $60 billion worth of patents on biological products are expiring before 2020, representing a major opportunity for the pharmaceutical industry. Market growth of biosimilars is expected to increase significantly, worth approximately $2 billion by 2018. While the prospect of cost savings and proficiency make the biosimilar market appealing, organizations and their outsourcing accomplices planning to enter this market must be aware of current regulations and issues in the global marketplace and be prepared to respond quickly to changes.
Related Conferences: Biosimilar Conferences | Biologics Workshops:
8th Annual Biosimilars Asia, May 15-18, 2017, Shanghai, China; World Biosimilar Congress, November 14-15, 2016, Basel, Switzerland; Biosimilars & Biobetters Congress, April 2017, London, UK; 3rd Biologics & Biosimilars Congress, March 6-7, 2017, Berlin, Germany; Biosimilars Europe Congress 2017, November 21-22, 2017, London, UK; 8th Global Pharmacovigilance & Drug Safety Summit, July 10-11, 2017 Jakarta, Indonesia; 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia; 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea; 11th International Conference on Pharmacoepidemiology and Clinical Research, July 06-08, 2017 Kuala Lumpur, Malaysia; 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia
BioSim-Asociación Española de Biosimilares , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association , IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 10: Globalization of Biosimilars
This track discuses about the generic drugs impact on global biosimilar market, Cost and risk management, Adopting innovative mechanisms such as risk-sharing arrangement, Asian market for biosimilars.
Those who can attend the biosimilar meeting under this track are ones following Biologics/Proteins/Biosimilar Products, New Biosimilar Development, Process Science, Biosimilar Market, Portfolio Management, Research & Development, Business Development, Business Operations and Scientific Affairs.
Related Conferences: Biosimilar Conferences | Biologics Conferences:
8th Annual Biosimilars Asia, May 15-18, 2017, Shanghai, China; World Biosimilar Congress, November 14-15, 2016, Basel, Switzerland; Biosimilars & Biobetters Congress, April 2017, London, UK; 3rd Biologics & Biosimilars Congress, March 6-7, 2017, Berlin, Germany; Biosimilars Europe Congress 2017, November 21-22, 2017, London, UK; 8th Global Pharmacovigilance & Drug Safety Summit, July 10-11, 2017 Jakarta, Indonesia; 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia; 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea; 11th International Conference on Pharmacoepidemiology and Clinical Research, July 06-08, 2017 Kuala Lumpur, Malaysia; 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia
BioSim-Asociación Española de Biosimilares , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association , IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 11: Biosimilars Analytical Strategies
Analysis of biosimilars and biologics forms to be one of the most important aspects towards the biologics and biosimilar development process. Biosimilars analytical methods for process development and validation as well as use of production technologies such as disposables and supply chain logistics can help companies establish facility flexibility.
This biosimilars global event also includes Bioanalytical methods, Formulation, Bioassay for comparability and potency testing, GMP protein analysis, LC/MS analysis for discovery, preclinical, and clinical programs.
Related Conferences: Biosimilar Conferences | Biologics Workshops:
8th Annual Biosimilars Asia, May 15-18, 2017, Shanghai, China; World Biosimilar Congress, November 14-15, 2016, Basel, Switzerland; Biosimilars & Biobetters Congress, April 2017, London, UK; 3rd Biologics & Biosimilars Congress, March 6-7, 2017, Berlin, Germany; Biosimilars Europe Congress 2017, November 21-22, 2017, London, UK; 8th Global Pharmacovigilance & Drug Safety Summit, July 10-11, 2017 Jakarta, Indonesia; 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia; 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea; 11th International Conference on Pharmacoepidemiology and Clinical Research, July 06-08, 2017 Kuala Lumpur, Malaysia; 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia
BioSim-Asociación Española de Biosimilares , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association , IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 12: Emerging Biosimilars in Therapeutics
The explorations in the field of biologics have created a new avenue for the clinicians towards better disease management. The emerging biologics have already manifested fruitful outcomes in treatment of ailments like those of psoriasis, rheumatic arthritis, certain cancers, inflammatory bowel disease (IBD) etc.
Emerging Biosimilar insulins are likely to enter the insulin landscape as patents for major branded insulin products start to expire in the next few years. The main theme of this track is to have sound knowledge in the emerging biosimilars like Recombinant blood products, therapeutic proteins, vaccines, Biosimilar antibodies, Growth hormones, Biosimilar peptides, therapeutic proteins and other biosimilar developments.
Related Conferences: Biologics Conferences | Biologics Meetings:
8th Annual Biosimilars Asia, May 15-18, 2017, Shanghai, China; World Biosimilar Congress, November 14-15, 2016, Basel, Switzerland; Biosimilars & Biobetters Congress, April 2017, London, UK; 3rd Biologics & Biosimilars Congress, March 6-7, 2017, Berlin, Germany; Biosimilars Europe Congress 2017, November 21-22, 2017, London, UK; 8th Global Pharmacovigilance & Drug Safety Summit, July 10-11, 2017 Jakarta, Indonesia; 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia; 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea; 11th International Conference on Pharmacoepidemiology and Clinical Research, July 06-08, 2017 Kuala Lumpur, Malaysia; 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia
BioSim-Asociación Española de Biosimilares , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association , IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 13: Clinical Studies on Biosimilars
This track includes Clinical trials on major diseases Risk management, and quality affairs, Case studies, and clinical models, Transgenic animals, Targeted cell line development, Clinical biosimilar tracks.docx PK/PD studies, Toxicological studies and Aspects of genotoxicity tests. This track is designed for those who are having sound knowledge on clinical studies and clinicians prospects for biosimilars. Biosimilar guidelines on the above mentioned topics are also to be thrown light upon at this biosimilars conference.
Related Conferences: Biosimilar Events | Biosimilars Conference:
8th Annual Biosimilars Asia, May 15-18, 2017, Shanghai, China; World Biosimilar Congress, November 14-15, 2016, Basel, Switzerland; Biosimilars & Biobetters Congress, April 2017, London, UK; 3rd Biologics & Biosimilars Congress, March 6-7, 2017, Berlin, Germany; Biosimilars Europe Congress 2017, November 21-22, 2017, London, UK; 8th Global Pharmacovigilance & Drug Safety Summit, July 10-11, 2017 Jakarta, Indonesia; 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia; 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea; 11th International Conference on Pharmacoepidemiology and Clinical Research, July 06-08, 2017 Kuala Lumpur, Malaysia; 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia
BioSim-Asociación Española de Biosimilares , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association , IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 14: BCS & IVIVC Based Biowavers
Biopharmaceutics Classification System and invitro and invivo classification discusses about ADME pathways of different drugs. This also includes BCS biowaivers, In vitro diffusion cells for dissolution testing in formulation development, In vitro preclinical ADME/ BCS testing. The objective of this work was to suggest the biowaivers potential of biopharmaceutical classification system which are known to increase the solubility, dissolution, oral absorption of water insoluble drugs. The Biosimilars Asia 2017 aims at addressing all such challenges of the pharma formulation sector for biobetters, biologics, biosimilars and biowaivers.
Related Conferences: Biowaivers Conferences | Biobetters Conferences:
8th Annual Biosimilars Asia, May 15-18, 2017, Shanghai, China; World Biosimilar Congress, November 14-15, 2016, Basel, Switzerland; Biosimilars & Biobetters Congress, April 2017, London, UK; 3rd Biologics & Biosimilars Congress, March 6-7, 2017, Berlin, Germany; Biosimilars Europe Congress 2017, November 21-22, 2017, London, UK; 8th Global Pharmacovigilance & Drug Safety Summit, July 10-11, 2017 Jakarta, Indonesia; 16th Annual Medicinal & Pharmaceutical Sciences Congress, July 03-05, 2017 Kuala Lumpur, Malaysia; 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea; 11th International Conference on Pharmacoepidemiology and Clinical Research, July 06-08, 2017 Kuala Lumpur, Malaysia; 9th World Congress on BA/BE Studies and Biowaivers, July 17-19, 2017 Melbourne, Australia
BioSim-Asociación Española de Biosimilares , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association , IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 15: Entrepreneurs Investment Meet
Entrepreneurs who are willing to put in hard work and invest in the field of biologics and biosimilars will find this meeting the best place to properly shape their drive for the new endeavours. Also this meeting will help them find the best experts who can make their investment fruitful and worthwhile. The best technological knowhow, economical aspects, regulatory red tapes and profit shares involving biosimilars and biologics shall be discussed at this Biosimilars Global Event.
The Asian Biosimilars 2017 is owned to bring together the worldwide top pharmaceuticals, biotechnology and regulatory representatives under one roof that will provide them the best platform to update and share knowledge related to biosimilars and biologics. Hence, the International Biosimilar Conference will unfold the multiple facets of biosimilars, ranging from the evolving regulatory landscapes, biosimilar guidelines to the legal and economic aspects and the then current challenges in biosimilar development. This biosimilar conference will focus on a variety of aspects of biosimilar product development to successful delivery of safe, efficacious and potent biosimilar products to the market. By attending this conference one will gain a comprehensive outlook on the key issues surrounding biosimilars as well as biologics. An important platform for Biosimilars stakeholders to discuss and share best practices in expediting development in the field of Biologics and Biosimilars is ought to be Asian Biosimilars 2017.
Welcome Message
Asian Biosimilars 2017

Dear Industry Colleagues, Scientists and Friends,
As a member of the Organizing Committee I am delighted to invite you to attend the 8th Asian Biologics and Biosimilars Congress to be held from August 10-12, 2017 in Beijing, China. The Asian Biosimilars Congress 2017 brings together biopharmaceutical industry colleagues, scientists, researchers, regulatory, and clinical experts from around the globe to bring to you the cutting edge information for the development of biosimilars.
The 8th Asian Biologics and Biosimilars Congress is designed to bring forth the most up to date information for the development of biosimilars. Key aspects will include regulatory, CMC, clinical and marketing considerations, as they relate to what it will take to bring these lifesaving/life altering products to the highly regulated Western Markets.
Please join your peers from around the world for this wonderful opportunity to reach the largest assembly of participants from the Biologics/Biosimilars community, meet with thought leadership experts, share your new research, and show off your cutting edge technology at this 3-day event.
I look forward to seeing you in Beijing, China at the 8th Asian Biologics and Biosimilars Congress.
Kamali Chance, MPH, PhD, RAC
Vice President
Head, Global Biosimilars Regulatory Strategy
Biosimilars Center of Excellence
QuintilesIMS
Welcome Message
Asian Biosimilars 2017



Dear Colleagues, Scientists and Friends,
The Organizing Committee is delighted to invite you to attend the 8th Asian Biosimilars Congress, one of its remarkable pharmaceutical conferences, to be held during August 10-12, 2017 in Beijing, China. The Asian Biosimilars Congress 2017 brings together scientists, researchers, industry and CRO representatives from around the world.
As of December 2016 there were 23 biosimilars marketed in the EU and 4 biosimilars licensed in the USA. As such, the biosimilars market is expected to experience an exponential growth over the coming few years. Many new biologics are making their entry in the pharma market and experiencing a notable rise in their usage compared with the conventional medications.
The 8th Asian Biosimilars Congress will present multiple aspects of biosimilars from the passage of biosimilars through a decade in Asia to the latest developments in the field of biologics and biosimilars. The whole range of portfolio selection, development, regulatory requirements and marketing will be presented.
You can join your peers from around the world focused on learning about biologics and biosimilars-related advances, which is your single best opportunity to reach the largest assembly of participants from the Biosimilars community, conduct demonstrations, distribute information, meet with professionals, make a splash with new research, and receive name recognition at this 3-day event.
The congress will provide enough space for discussion, meetings and workshops in order to allow a close contact between speakers and visitors.
We are looking forward to see you in Beijing, China
Raymond Huml, MS, DVM, RAC, QuintilesIMS
Oxana Iliach, PhD, QuintilesIMS
Charu Manaktala, MD, QuintilesIMS

Welcome Message Asian Biosimilars 2017
Dear Friend and Colleague,
On behalf of the Organizing Committee, it will be my great pleasure to welcome you to Beijing and to the Asian Biosimilars 2017.
The development of biosimilars is increasing at a rapid pace in all global regions, including the US with FDA’s approval of multiple biosimilars due to their potential for improving access to effective biological therapies through reduced costs; biosimilars have garnered great interest among industry, regulators, and payers.
This conference is unique in setting the stage for an open, collaborative discussion of important topics related to biosimilar drug development among global representatives from industry, academia, and regulatory agencies. The congress has been designed to provide an innovative and comprehensive overview of the latest research developments in Biosimilars; the current state of Biosimilars and prospects for the future, totality of the evidence, analytics, clinical considerations, education, real-world evidence, access, the science of biosimilars, and closing with a call to action.
This conference will also feature a special session on “Biosimilars Safety and Risk Management”.
I hope you will take advantage of the many opportunities to actively engage in discussions and with each other.
I am sure you will enjoy the conference and that your interaction with your colleagues from many different countries will stimulate a creative exchange of ideas and will be personally rewarding. I also hope and trust that you will enjoy your visit to the very beautiful and exciting city of Beijing.
Yours sincerely,
Asif Mahmood, MD, MPH, MBA
Disease Area Cluster Lead (Biosimilars & Drug Delivery Devices),
Safety Surveillance and Risk Management
Pfizer Worldwide Safety
The global Biosimilars market (Follow-On-Biologics) is expected to reach $26.5Billion by 2020 growing at a CAGR of 49.1% from 2015 to 2020 whereas The global biosimilars market alone is expected to reach $6.22 Billion by 2020 from $2.29 Billion in 2015, at a compound annual growth rate (CAGR) of 22.1% from 2015 to 2020.
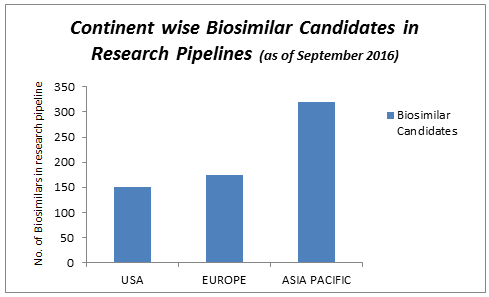
Geographically, the global biosimilars market is dominated by Asia, followed by Europe, Rest of the World (RoW), and North America. However, the Asia-Pacific region is likely to witness the highest growth rate during the forecast period.
With the biosimilar regulatory pathway insight in markets around the world, companies from the biopharma innovators to traditional small molecule generic houses to well-funded start-ups are exploring how they can enter the biosimilar business.
Countries in Asia, particularly India, Singapore, Korea, Japan and China, are the attractive locations to build bio development and bio manufacturing capabilities.
Benefits for establishing Biosimilar Companies in Asia:
- The resources and infrastructure are already in place in these countries.
- The current industry infrastructure, investment incentives and regulatory framework, and key participants in India, Singapore, Korea and China are promising.
- In addition, speakers’ profiled examples of companies are already building their biologics capabilities in these Asian countries.
- Asia can be a launch pad for biosimilars because of one, supportive governments.
-
And lastly, these countries have a developed clinical infrastructure.
There are currently more biosimilar products in development across the Asia-Pacific region than anywhere else in the world, leading to a wealth of opportunities for investigators and patients to take part in biosimilar clinical trials.
A recent report has shown that biosimilars in India have witnessed nearly 20% annual growth for the last financial year and now make up for about 2.5% of the overall biologics market.
- South Korea was the first country worldwide to approve biosimilar versions of etanercept according to international standards. In addition, the country’s regulators are currently working on class-specific biosimilar regulatory guidelines.
- Australia, as of August 2016, has approved 11 biosimilars (based on six originators) – second only to the EU. Australia is the world’s first highly regulated market to allow pharmacy level substitution of a monoclonal antibody biosimilar for an originator.
- As of April 2016, eight biosimilars have been approved in Japan, including two insulin glargine biosimilars.
Substantial price reductions for biosimilars have been seen within the Asia-Pacific region:
- In Japan and South Korea, formal price discount requirements for biosimilars generally range from 30–50% compared with the originator product.
- In South Korea, biosimilar competition is also driving down the price of originator products, with the price of the original reference product automatically dropping to 70% of its original market price as soon as the first biosimilar product is introduced into the market.
- Price reductions of more than one-third of the originator price have been seen with the introduction of recent biosimilars for rheumatoid arthritis in India.
- In China, a biosimilar to insulin glargine was introduced in 2013 (prior to the introduction of China’s official guidelines) with a price reduction of 26% compared with the originator.
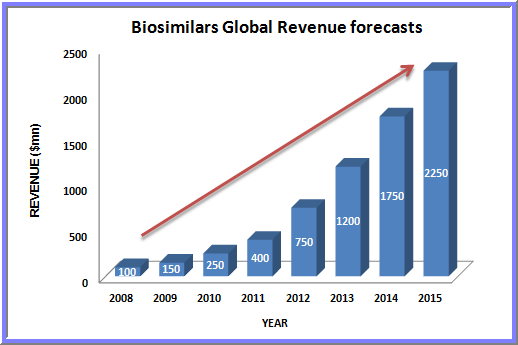
A number of factors such as growing pressure to curtail healthcare expenditure, growing demand of biosimilar drugs due to their cost effectiveness, rising incidences of various diseases, increasing number of off-patented drugs, positive outcome in the ongoing clinical trials, and rising demand for biosimilars in different therapeutic applications such as rheumatoid arthritis and blood disorders are propelling the growth of the global market.
The Organizing Committee is delighted to invite you to attend the 8th Asian Biologics and Biosimilars Congress one of its remarkable Pharmaceutical conferences, to be held during August 10-12, 2017 at Beijing, China. Asian Biosimilars Congress 2017 brings together scientists, researchers and CROs from around the world. With the approval of second biosimilar by the USFDA-Inflectra, the biosimilars market is owned to experience an exponential growth over the coming few years. Many biologics products are making their entry in the pharma market and experiencing a notable rise in their usage over the conventional medications.
At Asian Biosimilars 2017 meet your target audiences from around the world focused on learning about biologics and biosimilars. This conference would be your single best opportunity to reach the largest assemblage of participants from the biologics and biosimilars community.
Why to attend???
Join your peers around the world focused on learning about Biologics and Biosimilars related advances, which is your single best opportunity to reach the largest assemblage of participants from the Biosimilars community, conduct demonstrations, distribute information, meet with current and potential professionals, make a splash with a new research works, and receive name recognition at this 3-day event. World-renowned speakers, the most recent research, advances, and the newest updates in Biologics and Biosimilars are hallmarks of this conference.
Target Audience:
- Directors, CEO’s of Organizations
- Business Development Managers
- Chief Scientific Officers
- R&D Researchers from Biosimilar and Biologics Industries
- Professors, Associate Professors, Assistant Professors
- PhD Scholars
- Patent Attorneys
- Intellectual Property Attorneys
- Investment Analysts
- Association, Association presidents and professionals
- Noble laureates in Health Care and Medicine
- Bio instruments Professionals
- Bio-informatics Professionals
- Software development companies
- Research Institutes and members
- Supply Chain companies
- Manufacturing Companies
- CRO and DATA management Companies
- Training Institutes
- Business Entrepreneurs
Audience Share:
- Industry 60%
- Academia 30%
- Others 10%
Conference Highlights
- Challenges in Developing Biosimilars
- Emerging Biosimilars in Therapeutics
- Biosimilars Analytical Strategies
- Globalization of Biosimilars
- Clinical Studies on Biosimilars
- BCS & IVIVC Based Biowaivers
- Pharmacovigilance of Biosimilars
- Latest Biosimilars in Asian Scenario
- Biosimilars- Hatch-Waxman Act & BPCI Act
- Biosimilars Research Pipeline
- Current Developments in The Field of Biosimilars
- Emerging Trends in Biosimilars
- Biosimilars Market- Challenges & Prospects
- Cost Analysis of Biosimilars
- Entrepreneurs Investment Meet
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | August 10-12, 2017 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | Day 3 |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Bioanalysis & Biomedicine
- Journal of Bioequivalence & Bioavailability
- Journal of Pharmaceutical Sciences & Emerging Drugs
Abstracts will be provided with Digital Object Identifier by