Theme: Biosimilars: Exploring and Expanding the Strategies in Pharmacy
Asian Biosimilars 2019
Biosimilars Conferences inviting you to attend Biosimilars Congress 2019 on July 26-27, 2019 Melbourne, Australia. The topic of the current year's gathering is Biosimilars: Exploring and Expanding the Strategies in Pharmacy which will give a worldwide stage to talk of present and future of Biosimilars.
Biosimilars Congress 2019 gathering will empower us to continue plunging further into both the examination of Biosimilar change and the business necessities for associations. From around the world, top Biosimilars and Biologics experts meet at the largest networking conference Biosimilars Congress 2019.
The Organizing Committee is satisfied to welcome you to attend the Biosimilars Congress 2019, one of its astonishing Pharmaceutical gatherings to be held on July 26-27, 2019 in Melbourne, Australia.
Our Conferences on Biosimilars joins scientists, authorities, and CROs from around the world. The Biosimilars Congress 2019 experiences an exponential improvement over a coming couple of years. Various Biologics things are affecting their passage in the pharma to publicize and experiencing a striking climb in their utilization over the standard remedies.
At Biosimilars 2019 meet your planned intrigue bunches from around the world focused on getting some answers concerning Biosimilars and Biologics. This gathering would be your single most obvious opportunity to accomplish the greatest accumulation of individuals from the Biosimilars and Biologics gathering.
Biosimilars Congress 2018 gather was a hit we could get learning from two specific gathering one that surpassed desires in the deliberate and collecting end and one that surpassed desires in the business and key end.
This year’s flagship event will gather top Europe Associations, Societies, Companies, Laboratories, Global regulators, Officials, Healthcare Actors as well as Industry leaders, to foster open exchange and debate on the role of the Biosimilars and Biologics sector in “Biosimilars Congress 2019 : Biosimilars: Exploring and Expanding the Strategies in Pharmacy”.
Why attend???
Join your peers around the world focused on learning about Biologics and Biosimilars related advances, which is your single best opportunity to reach the largest assemblage of participants from the Biosimilars community, conduct demonstrations, distribute information, meet with current and potential professionals, make a splash with new research works, and receive name recognition at this 2-day event. World-renowned speakers, the most recent research, advances, and the newest updates in Biologics and Biosimilars are hallmarks of this conference.
Target Audience:
- Directors, CEO’s of Organizations
- Business Development Managers
- Chief Scientific Officers
- R&D Researchers from Biosimilar and Biologics Industries
- Professors, Associate Professors, Assistant Professors
- Ph.D. Scholars
- Patent Attorneys
- Intellectual Property Attorneys
- Investment Analysts
- Association, Association presidents and professionals
- Noble laureates in Health Care and Medicine
- Bio instruments Professionals
- Bio-informatics Professionals
- Software development companies
- Research Institutes and members
- Supply Chain companies
- Manufacturing Companies
- CRO and DATA management Companies
- Training Institutes
- Business Entrepreneurs
Audience Share:
- Industry 60%
- Academia 30%
- Others 10%
Track 1: Biosimilars Analytical Strategies
Analysis of biosimilars and biologics forms to be one of the most important aspects of the biologics and biosimilar development process. Biosimilars analytical methods for process development and validation as well as the use of production technologies such as disposables and supply chain logistics can help companies establish facility flexibility.
This biosimilars global event also includes Bioanalytical methods, Formulation, Bioassay for comparability and potency testing, GMP protein analysis, LC/MS analysis for discovery, preclinical, and clinical programs.
Related Conferences: Biosimilar Events | Biosimilar Conferences
13th Asian Biologics and Biosimilars Congress, July 26-27, 2019 Melbourne, Australia; 13th International Conference on Biosimilars and Biologics, November 14-15, 2018 Lisbon | Portugal; 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018 | Philadelphia, Pennsylvania, USA; 6 th European Biopharma Congress, September 18-19, 2018 Amsterdam, Netherlands; International Conference on Biological & Pharmaceutical Sciences November 19-20 ,2018 Sydney, Australia; International Conference on Computational Biology and Bioinformatics, September 05-06 2018 Tokyo, Japan; Analytical & Bioanalytical Techniques, September 19-20, 2018 Singapore;
BioSim-Asociación Española de Biosimilars, Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association, IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 2: Bioequivalence Assessment
Bioequivalence focuses on the equivalence of release of the active pharmaceutical ingredient from the pharmaceutical product and its subsequent absorption into the systemic circulation. This session has utmost importance in context to the fact that only a suitably bioequivalent drug candidate that confirms the results in all respects to the original licensed product can be called a biosimilar drug.
Of all attempts towards developing a follow on biologics or a biosimilar drug, the main detection point stands at the bioequivalence assessment. Once the bioequivalence has been obtained it can be 70% ascertained the drug qualifies to be a suitable biologics or biosimilars.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
13th Asian Biologics and Biosimilars Congress, July 26-27, 2019 Melbourne, Australia; 13th International Conference on Biosimilars and Biologics, November 14-15, 2018 Lisbon | Portugal; 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018 | Philadelphia, Pennsylvania, USA; 6 th European Biopharma Congress, September 18-19, 2018 Amsterdam, Netherlands; International Conference on Biological & Pharmaceutical Sciences November 19-20 ,2018 Sydney, Australia; International Conference on Computational Biology and Bioinformatics, September 05-06 2018 Tokyo, Japan; Analytical & Bioanalytical Techniques, September 19-20, 2018 Singapore;
BioSim-Asociación Española de Biosimilars, Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association, IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 3: Regulatory Approach for Biosimilars
Biosimilars are the generic version of biological. It is the new buzz word in the pharmaceutical industry. Biosimilars are highly similar to the licensed reference product notwithstanding minor differences in clinically inactive components; also there are no clinically meaningful differences between the biologicals and the reference product in terms of safety, purity, and potency. This track includes Licensing of biosimilars, Biosimilars regulation, Patent issues, BLA filing for biosimilars, Biosimilars regulatory prospects of BRIC countries, a paradigm of traditional generics to Biosimilars, Biowaiver approval for Biosimilars and other aspects of Biosimilar approvals. Biosimilars 2018 will provide an excellent and global opportunity to the scientists, partners and pharma leaders from Biopharmaceutical and Biotechnology industries to innovate and to explore the strategic market for Biosimilars and Biologics with a clear picture of the regulatory approach for biosimilars and biologics.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
13th Asian Biologics and Biosimilars Congress, July 26-27, 2019 Melbourne, Australia; 13th International Conference on Biosimilars and Biologics, November 14-15, 2018 Lisbon | Portugal; 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018 | Philadelphia, Pennsylvania, USA; 6 th European Biopharma Congress, September 18-19, 2018 Amsterdam, Netherlands; International Conference on Biological & Pharmaceutical Sciences November 19-20 ,2018 Sydney, Australia; International Conference on Computational Biology and Bioinformatics, September 05-06 2018 Tokyo, Japan; Analytical & Bioanalytical Techniques, September 19-20, 2018 Singapore;
BioSim-Asociación Española de Biosimilars, Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association, IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 4: Challenges in Biosimilars Pharmacovigilance
This session of the Biosimilars 2018 looks into the future and FDA initiatives that have already been announced to include enhanced tracking and follow-up of postmarket issues planned improvements in AERS and pilots of new post-market drug-monitoring strategies. Current challenges in pharmacovigilance, Adverse drug reactions with pharmaceutical products, Biosimilar guidelines for pharmacovigilance practice and pharmacoepidemiology are the points that shall be laid emphasis in this session.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
13th Asian Biologics and Biosimilars Congress, July 26-27, 2019 Melbourne, Australia; 13th International Conference on Biosimilars and Biologics, November 14-15, 2018 Lisbon | Portugal; 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018 | Philadelphia, Pennsylvania, USA; 6 th European Biopharma Congress, September 18-19, 2018 Amsterdam, Netherlands; International Conference on Biological & Pharmaceutical Sciences November 19-20 ,2018 Sydney, Australia; International Conference on Computational Biology and Bioinformatics, September 05-06 2018 Tokyo, Japan; Analytical & Bioanalytical Techniques, September 19-20, 2018 Singapore;
BioSim-Asociación Española de Biosimilars, Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association, IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 5: Clinical Development Of Biosimilars
This track includes Clinical trials on major diseases Risk management, and quality affairs, Case studies, and clinical models, Transgenic animals, Targeted cell line development, PK/PD studies, Toxicological studies, ethics maintained in clinical and preclinical studies, development difficulties and Aspects of genotoxicity tests. Biosimilar guidelines on the above-mentioned topics are also to be thrown light upon at this biosimilars conference.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
13th Asian Biologics and Biosimilars Congress, July 26-27, 2019 Melbourne, Australia; 13th International Conference on Biosimilars and Biologics, November 14-15, 2018 Lisbon | Portugal; 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018 | Philadelphia, Pennsylvania, USA; 6 th European Biopharma Congress, September 18-19, 2018 Amsterdam, Netherlands; International Conference on Biological & Pharmaceutical Sciences November 19-20 ,2018 Sydney, Australia; International Conference on Computational Biology and Bioinformatics, September 05-06 2018 Tokyo, Japan; Analytical & Bioanalytical Techniques, September 19-20, 2018 Singapore;
BioSim-Asociación Española de Biosimilars , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association, IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 6: Biosimilars & Biologics: Clinical Studies & Trials
This track includes Clinical Studies & Trials on major diseases Risk management, and quality affairs, Case studies, and clinical models, Transgenic animals, Targeted cell line development, Toxicological studies and Aspects of genotoxicity tests. Clinical trials are designed in stages I-IV so as to receive a clear picture of the drug candidate in respect to its pharmacokinetics and pharmacodynamics parameters. Research estimates that there are 280 Biosimilars in the pipeline, and clinical trials are increasing by 20% per year. Biologics also represent over 40 percent of the drugs in each of the preclinical, Phase I, Phase II, and Phase III trial stages.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
13th Asian Biologics and Biosimilars Congress, July 26-27, 2019 Melbourne, Australia; 13th International Conference on Biosimilars and Biologics, November 14-15, 2018 Lisbon | Portugal; 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018 | Philadelphia, Pennsylvania, USA; 6 th European Biopharma Congress, September 18-19, 2018 Amsterdam, Netherlands; International Conference on Biological & Pharmaceutical Sciences November 19-20 ,2018 Sydney, Australia; International Conference on Computational Biology and Bioinformatics, September 05-06 2018 Tokyo, Japan; Analytical & Bioanalytical Techniques, September 19-20, 2018 Singapore;
BioSim-Asociación Española de Biosimilars , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association, IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 7: Biosimilars & Biologics: Current Challenges
The generic version of Biologicals- “Biosimilars” is the new buzz word in the world of the pharmaceutical industry. Biosimilars are highly similar to their licensed reference product notwithstanding minor differences as excipients in the formulation; also there are no remarkable differences between the Biologicals and the reference product in terms of safety, purity, and potency. However, there are certain challenges in way of its development and receiving a green signal for launching into the market. Newer biologics also are targeting widespread diseases, with profound implications: a drug that costs $20,000 per year that is useful for 1 person in 100,000 has much less effect on a health plan’s cost structure than a $5,000-per-year drug that is useful for 1 in 100 people.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
13th Asian Biologics and Biosimilars Congress, July 26-27, 2019 Melbourne, Australia; 13th International Conference on Biosimilars and Biologics, November 14-15, 2018 Lisbon | Portugal; 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018 | Philadelphia, Pennsylvania, USA; 6 th European Biopharma Congress, September 18-19, 2018 Amsterdam, Netherlands; International Conference on Biological & Pharmaceutical Sciences November 19-20 ,2018 Sydney, Australia; International Conference on Computational Biology and Bioinformatics, September 05-06 2018 Tokyo, Japan; Analytical & Bioanalytical Techniques, September 19-20, 2018 Singapore;
BioSim-Asociación Española de Biosimilars , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association, IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 8: Biosimilars: Pharmacovigilance and Safety
Biosimilar guidelines for Pharmacovigilance practice and Pharmacoepidemiology are the points that shall be laid emphasis in this session. U.S. average annual spending growth from 2002 to 2007 was 16% for biologics, compared with 3.7% for drugs. In same proportion Pharmacovigilance for Biosimilars has been comparatively more than other pharmaceutical products. Biosimilars are interlinked with FDA activities that have as of now been declared to incorporate upgraded following and follow-up of postmarket issues, arranged changes in AERS, and pilots of new post advertise medicate observing systems. Current difficulties in Pharmacovigilance, adverse medication responses with pharmaceutical items, Biosimilar rules for Pharmacovigilance practice and pharmacoepidemiology are the focuses that should be laid accentuation in this session.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
13th Asian Biologics and Biosimilars Congress, July 26-27, 2019 Melbourne, Australia; 13th International Conference on Biosimilars and Biologics, November 14-15, 2018 Lisbon | Portugal; 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018 | Philadelphia, Pennsylvania, USA; 6 th European Biopharma Congress, September 18-19, 2018 Amsterdam, Netherlands; International Conference on Biological & Pharmaceutical Sciences November 19-20 ,2018 Sydney, Australia; International Conference on Computational Biology and Bioinformatics, September 05-06 2018 Tokyo, Japan; Analytical & Bioanalytical Techniques, September 19-20, 2018 Singapore;
BioSim-Asociación Española de Biosimilars , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association, IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 9: Globalization of Biosimilars
Globalization of Biosimilars is about the generic drug's impact on Global Biosimilar market, Cost and risk management, adopting innovative mechanisms such as risk-sharing arrangement, the European market for Biosimilars. A growing global market for Biosimilars is gaining momentum in response to the expiration of patents for a number of key biologics and consumer demand to reduce treatment costs. Thus, according to Research and Markets, the global biosimilar market, valued at $2 billion in 2012 is projected to reach $19.4 billion by 2018.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
13th Asian Biologics and Biosimilars Congress, July 26-27, 2019 Melbourne, Australia; 13th International Conference on Biosimilars and Biologics, November 14-15, 2018 Lisbon | Portugal; 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018 | Philadelphia, Pennsylvania, USA; 6 th European Biopharma Congress, September 18-19, 2018 Amsterdam, Netherlands; International Conference on Biological & Pharmaceutical Sciences November 19-20 ,2018 Sydney, Australia; International Conference on Computational Biology and Bioinformatics, September 05-06 2018 Tokyo, Japan; Analytical & Bioanalytical Techniques, September 19-20, 2018 Singapore;
BioSim-Asociación Española de Biosimilars , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association, IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 10: Innovation and Technology for Biosimilar Development
A biosimilar is an organic item very like an affirmed natural item, known as a kind of perspective item, with no clinically significant contrasts as far as wellbeing and viability. In the U.S., if a natural compound exhibits practically identical information to a U.S. Nourishment and Drug Administration (FDA)- authorized item from expository, preclinical and clinical reviews, it will be acknowledged as a biosimilar after termination of trend-setter licenses through a curtailed course. Tradable natural items are likewise Biosimilars, however, should meet extra criteria to coordinate the reference item. Interchangeable can be substituted for the reference item without medicine from a social insurance supplier.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
13th Asian Biologics and Biosimilars Congress, July 26-27, 2019 Melbourne, Australia; 13th International Conference on Biosimilars and Biologics, November 14-15, 2018 Lisbon | Portugal; 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018 | Philadelphia, Pennsylvania, USA; 6 th European Biopharma Congress, September 18-19, 2018 Amsterdam, Netherlands; International Conference on Biological & Pharmaceutical Sciences November 19-20 ,2018 Sydney, Australia; International Conference on Computational Biology and Bioinformatics, September 05-06 2018 Tokyo, Japan; Analytical & Bioanalytical Techniques, September 19-20, 2018 Singapore;
BioSim-Asociación Española de Biosimilars , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association, IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 11: Biopharmaceuticals
A biopharmaceutical is likewise called as a Biological or Biologic pharmaceutical medicine thing manufactured from natural sources through extraction. The progression of pharmaceutical medicine regularly takes a typical of 10 – 15 years. Such a lot of effort, time and money, it is particularly pressing that the right headways and materials are gotten in the examination, change and amassing of the pharmaceutical solutions, entire process consolidates pre-exposure, pre-clinical trials, exhibit dispatch to post advancing watching. The wellsprings of prescription things fuse peptides and proteins, including monoclonal antibodies and neutralizer pieces. From 2000 to 2006, biologics spoke to 33% of all New Active Substances that were pre moved, and are reflected in the present headway pipelines of the pharmaceutical business.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
13th Asian Biologics and Biosimilars Congress, july 26-27, 2019 Melbourne, Australia; 13th International Conference on Biosimilars and Biologics, November 14-15, 2018 Lisbon | Portugal; 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018 | Philadelphia, Pennsylvania, USA; 6 th European Biopharma Congress, September 18-19, 2018 Amsterdam, Netherlands; International Conference on Biological & Pharmaceutical Sciences November 19-20 ,2018 Sydney, Australia; International Conference on Computational Biology and Bioinformatics, September 05-06 2018 Tokyo, Japan; Analytical & Bioanalytical Techniques, September 19-20, 2018 Singapore;
BioSim-Asociación Española de Biosimilars , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association, IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 12: Biologics and Biosimilars
The development of biologics calls for overcoming lot many challenges. With initial steps of concepts of biologics, their considerations, essentials for early clinical developments it is very much needed that proper scientific and strategic approaches are taken for the successful development of follow-on-biologics. Moreover, the need for overcoming the challenges continues in the late clinical steps, drug safety factors, and labeling requirements. Also, it is much required now to develop a drug product in accordance with quality by design (QbD). This Euro Biopharma 2017 conference will look at the multiple facets of current challenges in biosimilar development. This conference will focus on multiple aspects of biosimilar product development to successfully deliver safe, potential and efficacious biologic products to the market.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
13th Asian Biologics and Biosimilars Congress, July 26-27, 2019 Melbourne, Australia; 13th International Conference on Biosimilars and Biologics, November 14-15, 2018 Lisbon | Portugal; 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018 | Philadelphia, Pennsylvania, USA; 6 th European Biopharma Congress, September 18-19, 2018 Amsterdam, Netherlands; International Conference on Biological & Pharmaceutical Sciences November 19-20 ,2018 Sydney, Australia; International Conference on Computational Biology and Bioinformatics, September 05-06 2018 Tokyo, Japan; Analytical & Bioanalytical Techniques, September 19-20, 2018 Singapore;
BioSim-Asociación Española de Biosimilars, Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association, IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 13: Pharmaceutical Regulatory Affairs And IPR
Good Manufacturing Practices quality of drugs is essentially the responsibility of manufacturers. GMP Guidelines are meant to assure this very quality of drugs. CGMP refers to the Current Good Manufacturing Practice regulations enforced by the US Food and Drug Administration (FDA). cGMPs provide for systems that assure proper design, monitoring, and control of manufacturing processes and facilities. Adherence to the cGMP regulations assures the identity, strength, quality, and purity of drug products by requiring that manufacturers of medications adequately control manufacturing operations. GMP is actually good common sense quality management quality assurance GMP production and quality control.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
13th Asian Biologics and Biosimilars Congress, July 26-27, 2019 Melbourne, Australia; 13th International Conference on Biosimilars and Biologics, November 14-15, 2018 Lisbon | Portugal; 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018 | Philadelphia, Pennsylvania, USA; 6 th European Biopharma Congress, September 18-19, 2018 Amsterdam, Netherlands; International Conference on Biological & Pharmaceutical Sciences November 19-20 ,2018 Sydney, Australia; International Conference on Computational Biology and Bioinformatics, September 05-06 2018 Tokyo, Japan; Analytical & Bioanalytical Techniques, September 19-20, 2018 Singapore;
BioSim-Asociación Española de Biosimilars , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association, IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
Track 14: Therapeutic Biological Products
Products that are obtained from specific organs or tissues said to correspond with the unhealthy organs or tissues of the recipient. Proponents claim that the recipient's body automatically transports the injected cells to the target organs, where they supposedly strengthen them and regenerate their structure. The organs and glands used in cell treatment include brain, pituitary, thyroid, adrenals, thymus, liver, kidney, pancreas, spleen, heart, ovary, testis, and parotid. Several different types of cell or cell extract can be given simultaneously - some practitioners routinely give up to 20 or more at once.
Related Conferences: Biosimilar Events | Biosimilar Conferences:
13th Asian Biologics and Biosimilars Congress, July 26-27, 2019 Melbourne, Australia; 13th International Conference on Biosimilars and Biologics, November 14-15, 2018 Lisbon | Portugal; 15th International Conference on Pharmaceutical Formulations & Drug Delivery, September 17-18, 2018 | Philadelphia, Pennsylvania, USA; 6 th European Biopharma Congress, September 18-19, 2018 Amsterdam, Netherlands; International Conference on Biological & Pharmaceutical Sciences November 19-20 ,2018 Sydney, Australia; International Conference on Computational Biology and Bioinformatics, September 05-06 2018 Tokyo, Japan; Analytical & Bioanalytical Techniques, September 19-20, 2018 Singapore;
BioSim-Asociación Española de Biosimilars , Medicines for Europe(EGA), GPhA-Generic Pharmaceutical Association, IGBA- International Generic and Biosimilars Medicines Association, BGMA-British Biosimilars Association
The global Biosimilars market (Follow-On-Biologics) is expected to reach $26.5Billion by 2020 growing at a CAGR of 49.1% from 2015 to 2020 whereas The global biosimilars market alone is expected to reach $6.22 Billion by 2020 from $2.29 Billion in 2015, at a compound annual growth rate( CAGR )of 22.1% from 2015 to 2020.
Geographically, the global biosimilars market is dominated by Europe, followed by Asia-Pacific, Rest of the World (RoW), and North America. However, the Asia-Pacific region is likely to witness the highest growth rate during the forecast period.
There are currently more biosimilar products in development across the Asia-Pacific region than anywhere else in the world, leading to a wealth of opportunities for investigators and patients to take part in biosimilar clinical trials.
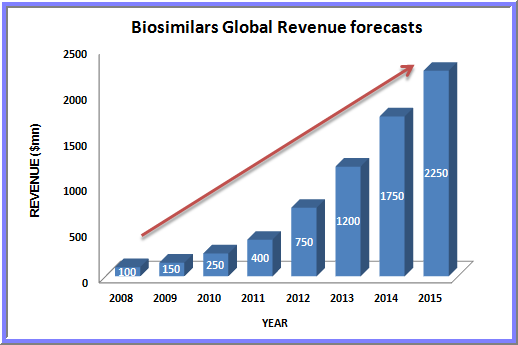
- South Korea was the first country worldwide to approve biosimilar versions of etanercept according to international standards. In addition, the country’s regulators are currently working on class-specific biosimilar regulatory guidelines.
- Australia, as of August 2016, has approved 11 biosimilars (based on six originators) – second only to the EU. Australia is the world’s first highly regulated market to allow pharmacy level substitution of a monoclonal antibody biosimilar for an originator.
- As of April 2016, eight biosimilars have been approved in Japan, including two insulin glargine biosimilars.
- A recent report has shown that biosimilars in India have witnessed nearly 20% annual growth for the last financial year and now make up for about 2.5% of the overall biologics market.
Substantial price reductions for biosimilars have been seen within the Asia-Pacific region:
- In Japan and South Korea, formal price discount requirements for biosimilars generally range from 30–50% compared with the originator product.
- In South Korea, biosimilar competition is also driving down the price of originator products, with the price of the original reference product automatically dropping to 70% of its original market price as soon as the first biosimilar product is introduced into the market.
- Price reductions of more than one-third of the originator price have been seen with the introduction of recent biosimilars for rheumatoid arthritis in India.
- In China, a biosimilar to insulin glargine was introduced in 2013 (prior to the introduction of China’s official guidelines) with a price reduction of 26% compared with the originator.
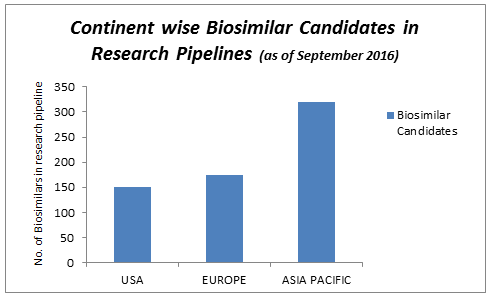
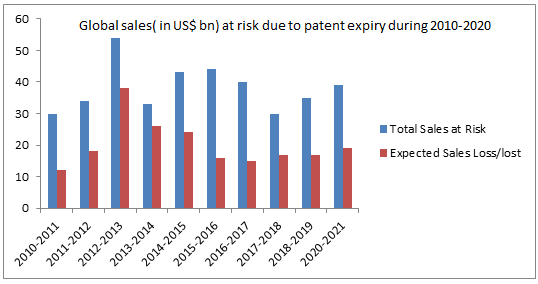
A number of factors such as growing pressure to curtail healthcare expenditure, growing demand of biosimilar drugs due to their cost effectiveness, rising incidences of various diseases, increasing number of off-patented drugs, positive outcome in the ongoing clinical trials, and rising demand for biosimilars in different therapeutic applications such as rheumatoid arthritis and blood disorders are propelling the growth of the global market.
Conference Highlights
- Biosimilars Analytical Strategies
- Bioequivalence Assessment
- Regulatory Approach for Biosimilars
- Biopharmaceuticals
- Challenges in Biosimilars Pharmacovigilance
- Clinical Development Of Biosimilars
- Globalization of Biosimilars
- Biosimilars & Biologics: Clinical Studies & Trials
- Biosimilars & Biologics: Current Challenges
- Biosimilars: Pharmacovigilance and Safety
- Innovation and Technology for Biosimilar Development
- Biologics and Biosimilars
- Pharmaceutical Regulatory Affairs And IPR
- Therapeutic Biological Products
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | July 26-27, 2019 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | |||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Bioanalysis & Biomedicine
- Journal of Bioequivalence & Bioavailability
- Journal of Pharmaceutical Sciences & Emerging Drugs
Abstracts will be provided with Digital Object Identifier by



























