Day 1 :
Keynote Forum
Arthur G. Cook
ZS Associates, USA
Keynote: The commercial landscape for biosimilars: Planning in an uncertain environment
Time : 10:15-11:05

Biography:
Arthur G Cook is a Principal at ZS Associates in San Francisco, CA. He is experienced in strategic planning, forecasting, market research, pricing, and decision analysis. He has worked with pharmaceutical companies across 25 countries in North America, Latin America, Asia, Europe and Australia, and has developed forecasts for products in over 150 different therapeutic areas. His experience prior to joining ZS Associates includes positions at two major pharmaceutical companies. He also has been a featured speaker at numerous pharmaceutical and health care industry conferences and is the author of the 2015 second edition book entitled “Forecasting for the Pharmaceutical Industry”. Currently, he heads the Biosimilars Center of Excellence at ZS Associates. He holds an MBA from the University of California at Berkeley, a PhD in Chemistry from Harvard University, and a BS in Chemistry from Stanford University.
Abstract:
Over the next 10 years $71 billion in worldwide biopharmaceutical revenue may be affected by the launch of biosimilar products. Several biosimilar launches already have taken place in multiple countries, and observations from uptake in these markets hold insight for strategic planners. In countries such as the US the commercial landscape is complicated by additional uncertainties in regulatory and legal policy. In this talk, we will examine the learnings from prior launches and apply these insights in a decision analysis framework to aid in planning for future biosimilar introductions.
Keynote Forum
Christoph Volpers
Michalski Hüttermann & Partner, Germany
Keynote: Intellectual property and regulatory interplay in biosimilars
Time : 11:30-12:20

Biography:
Christoph Volpers has done his PhD in Molecular Biology from the University of Mainz, Germany, and an MBA from Bradford University, UK. He has almost 15 years of experience in Intellectual Property and Licensing. Before he joined the patent law firm, Michalski Hüttermann & Partners, Dusseldorf/Munich, as Senior Patent Consultant in early 2015, he was the Director IP Biologics of the Teva Pharmaceutical Industries for six years with global responsibility for innovative biologics and biosimilar products. He is the Founder of a biopharmaceutical consultancy firm, author of more than 20 publications and a member of the Licensing Executive Society.
Abstract:
Driven by strong, albeit diverging interests of various stakeholders like pharmaceutical companies, legislators, health care providers and patient interest groups, the global biosimilar market is expected to reach more than US-$ 40 billion by mid of the next decade. Whereas, the regulatory pathway for biosimilars in Europe has been validated and refined over the last ten years, the US is still struggling for guidance and interpretation of its biosimilars legislation where regulatory and patent issues are much more connected. The talk will give an update on recent case law in this field and discuss strategic implications from various perspectives.
- Innovative Approach for Biosimilars | Clinical Development of Biosimilars | Emerging Biosimilars in Therapeutics
Location: Lilli Palmer
Session Introduction
Ulrike Konrad
Protagen Protein Services, Germany
Title: CMC considerations for biosimilar drug development
Time : 13:35-14:15

Biography:
Ulrike Konrad has more than 10 years of experience in Biotech/Pharma with focus on protein analytics and biosimilar development to reach and demonstrate biosimilarity. She graduated in Chemistry from the University in Heidelberg, Germany and has done her Master of Business Administration. She has worked as a Project Manager in different Biotech companies and joined Protagen Protein Services in 2012. In her role, she has supported numerous biosimilar developments including the consulting for the analytical and regulatory strategy for Europe, USA, India, Korea, Brazil and Mexico.
Abstract:
Developing a biosimilar from a CMC perspective is a scientific and risk based approach and not a list with checkboxes to be ticked off. Following the quality strategy, the biosimilarity cannot be tested in the clinical trials but must be demonstrated on the drug substance and product level. For this, there are typical road maps always starting with a quality target product profile of the originator molecules. This talk will focus on typical analytical modules on the road map also touching the comparison of degradation profiles and impurities of biosimilar and originator molecules in a side-by-side analytical situation. All that must be adapted to the company’s strategy for market, time for development, budget and partners. Typical examples will be presented combined with pitfalls that companies are facing during the development.
Stanley Seung Suh Hong
Celltrion Healthcare Co. Ltd., South Korea
Title: Evidence-based approach of CT-P13 to meet the expectations of different stakeholders
Time : 14:15-14:55

Biography:
Stanley Seung Suh Hong is a Senior Adviser at the Celltrion Healthcare and has played an important part in its development and success. He was the President of Research and Development at Celltrion, Inc., where he was responsible for the entire R&D including product discovery as well as biosimilar development. His team led the successful development of REMSIMA™, the world’s first biosimilar, and gained approval for the product in Korea, Japan, Canada, European Union and USFDA. He was also responsible for the development of other biosimilars in Celltrion, and has presented data on biosimilars at national and international medical meetings. He was also the President and CEO of Celltrion Healthcare.
Abstract:
Background: CT-P13, a biosimilar of infliximab, is the world’s first biosimilar monoclonal antibody approved in the USA, EU, Japan and many other countries for the treatment of autoimmune diseases. Biosimilars are known to have the potential to improve patient access and reduce healthcare costs. To capture these benefits, there has been a growing trend to encourage the use of biosimilars among health authorities. The National Institute for Health and Care Excellence of the United Kingdom has recommended choosing the least expensive treatment option for RA and UC, and the Italian Medicines Agency published a report on the opportunities laid by biosimilars in addressing the sustainability of care, for instance. However, other stakeholders including physicians and patients, in contrast, showed reluctance to this move due to concerns on safety and efficacy of biosimilars.
Approach: To reassure various stakeholders, CT-P13 has taken evidence based approach and has been generating data to solve most concerned area in the use of biosimilars, which are biosimilarity, extrapolation, and switching. As of September 2016, 7,759 patients were treated with CT-P13, and the efficacy and safety of CT-P13 including those in switching condition were observed and compared with its reference group.
Conclusion & Significance: Based on the positive real world evidence and clinical data supporting biosimilarity, extrapolation, and switching of CT-P13, there was an evolution of perception. This positive change has consequently resulted in the increased acceptance towards CT-P13 and fast uptake, enabling greater access to infliximab treatment via reallocation of healthcare budget.

Biography:
Tim Demuth, MD is a Clinical Development Unit Head at Sandoz Biopharma responsible for clinical strategy, and execution of biosimilar development programs. He has extensive experience in early and late stage drug development across multiple therapeutic areas including immunology, hematology and oncology. He has great passion for innovative approaches to drug development with the aim to improve patient access to medicines.
Abstract:
GP2015 has been developed as a biosimilar to Enbrel® (etanercept) and was approved as Erelzi by US FDA in August 2016. Assessment of biosimilarity follows the totality-of-the-evidence concept taking into consideration physicochemical, biological, nonclinical and clinical data. Biosimilarity to the approved originator product is confirmed in a step-wise approach. Characterization of multiple originator batches was conducted over time followed by an iterative, target-directed development process to yield a product falling into the variability range of the originator. Biosimilarity between GP2015 and the originator was confirmed at analytical, functional, nonclinical, pharmacokinetic (PK) and clinical levels. Bioequivalence between GP2015 and the originator was assessed in PK studies in animals and healthy volunteers. To detect clinically meaningful differences between GP2015 and originator, efficacy, safety and immunogenicity was studied in patients with moderate to severe chronic plaque type psoriasis. This phase III confirmatory study assessed the Psoriasis Area and Severity Index response rate at weeks 12, 30 and 52. ErelziTM was developed at the same dosage and strength as the originator. Multiple analytical methods showed high similarity between GP2015 and the originator. The aminoacid sequence was confirmed to be identical and protein folding was indistinguishable. In-vitro assays showed GP2015 and the originator had similar bioactivity. PK bioequivalence between GP2015 and the originator was established in nonclinical and human studies. The study in patients with psoriasis confirmed similar efficacy and safety and comparable immunogenicity in a highly sensitive indication. In all studies, no clinically meaningful differences between GP2015 and the originator were observed. Analytical, functional, nonclinical and clinical data provide comprehensive understanding of GP2015 and the originator and demonstrate a high level of similarity between the two substances in accordance with regulatory requirements. The totality of evidence of biosimilarity justifies the use of the biosimilar in the same indications as Enbrel® and was confirmed by FDA.
Dina E Abo Elezz
Pharos University, Egypt
Title: Development and in-vivo evaluation of Ofloxacin gastro-retentive drug delivery system
Time : 16:05-16:45
Biography:
Dina E Abo Elezz works as an Assistant Lecturer in the Faculty of Pharmacy, Pharos University in Alexandria, Egypt. She has done her Master’s degree in Pharmaceutical Sciences from Cairo University, Egypt. She is experienced in research and teaching. Her research interests are in pharmaceutical sciences, pharmacokinetics, bioavailability, bioequivalence and biosimilar.
Abstract:
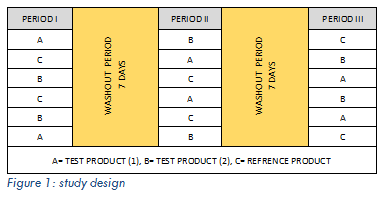
Ofloxacin is readily soluble in the acidic environment of the stomach, following oral administration; there is rapid and extensive oral absorption from the gastrointestinal tract. Gastric retention is designed to prolong gastric residence time of oral controlled release dosage forms which enhance therapeutic efficacy, reduce time intervals for drug administration, potentially reduced dose size and thus improved patient compliance. Factorial designs of 23 were planned for the optimization of ofloxacin floating and size increasing tablets, using Design-Expert® software. Convenient parameters which measures the floating lag time, floating duration, size increasing behavior, t50% and drug release tests were performed in 0.1 N HCl at 37ºC of the suggested formulations of the design, where different concentrations of different polymers (HPMC K100M, HPMC K15M and PEO wsr303) were used. Responses of the floating preparation were principally related to the polymer type rather than polymer concentration, while responses of the size increasing preparation were related to both polymer type and concentration. Comparative randomized, single dose, three-way crossover open label study was done to evaluate in vivo release and pharmacokinetics parameters of two different ofloxacin gastroretentive tablets 400 mg/tablet (test product 1, 2) relative to Tarivid® conventional tablet (2x200 mg/tablet) (reference product) after single oral administration to six healthy adult males as shown in Figure 1. Drugs were administered to fasted volunteers and blood samples were collected up to 24 hours and assayed for ofloxacin using a validated LC-MS/MS method. The pharmacokinetic parameters AUC0-t, AUC0-∞, Cmax, Tmax, T1/2, MRT and elimination rate constant were determined from plasma concentration-time profile by non-compartmental analysis method.
Arthur G. Cook
ZS Associates, USA
Title: The commercial landscape for biosimilars: Planning in an uncertain environment

Biography:
Arthur G Cook is a Principal at ZS Associates in San Francisco, CA. He is experienced in strategic planning, forecasting, market research, pricing, and decision analysis. He has worked with pharmaceutical companies across 25 countries in North America, Latin America, Asia, Europe and Australia, and has developed forecasts for products in over 150 different therapeutic areas. His experience prior to joining ZS Associates includes positions at two major pharmaceutical companies. He also has been a featured speaker at numerous pharmaceutical and health care industry conferences and is the author of the 2015 second edition book entitled “Forecasting for the Pharmaceutical Industry”. Currently, he heads the Biosimilars Center of Excellence at ZS Associates. He holds an MBA from the University of California at Berkeley, a PhD in Chemistry from Harvard University, and a BS in Chemistry from Stanford University.
Abstract:
Over the next 10 years $71 billion in worldwide biopharmaceutical revenue may be affected by the launch of biosimilar products. Several biosimilar launches already have taken place in multiple countries, and observations from uptake in these markets hold insight for strategic planners. In countries such as the US the commercial landscape is complicated by additional uncertainties in regulatory and legal policy. In this talk, we will examine the learnings from prior launches and apply these insights in a decision analysis framework to aid in planning for future biosimilar introductions.
Christoph Volpers
Michalski Hüttermann & Partner, Germany
Title: Intellectual property and regulatory interplay in biosimilars

Biography:
Christoph Volpers has done his PhD in Molecular Biology from the University of Mainz, Germany, and an MBA from Bradford University, UK. He has almost 15 years of experience in Intellectual Property and Licensing. Before he joined the patent law firm, Michalski Hüttermann & Partners, Dusseldorf/Munich, as Senior Patent Consultant in early 2015, he was the Director IP Biologics of the Teva Pharmaceutical Industries for six years with global responsibility for innovative biologics and biosimilar products. He is the Founder of a biopharmaceutical consultancy firm, author of more than 20 publications and a member of the Licensing Executive Society.
Abstract:
Driven by strong, albeit diverging interests of various stakeholders like pharmaceutical companies, legislators, health care providers and patient interest groups, the global biosimilar market is expected to reach more than US-$ 40 billion by mid of the next decade. Whereas, the regulatory pathway for biosimilars in Europe has been validated and refined over the last ten years, the US is still struggling for guidance and interpretation of its biosimilars legislation where regulatory and patent issues are much more connected. The talk will give an update on recent case law in this field and discuss strategic implications from various perspectives.