Day 2 :
Keynote Forum
Ulrich Storz
Michalski Hüttermann & Partner, Germany
Keynote: How AbbVie tries to fend off world’s blockbuster No 1 from generic competition
Time : 10:15-11:05

Biography:
Ulrich Storz received his PhD from the University of Münster in 2002 with a thesis in neurobiology. He then completed his training to become a German patent attorney. He joined the list of professional representatives before the European Patent Office in 2006. Dr. Storz's main areas of practice are managing and enforcing patents and patent applications as well as drafting FTO analyses and opinions. He also provides advice on strategic patent issues, notably in life sciences (biotechnology, biophysics, biochemistry and microbiology), above all in the field of therapeutic antibodies. Ulrich Storz is regularly involved in major antibody opposition cases before the European Patent Office. In recent years, he has also provided representation in a large number of sizeable due diligence projects in the field of pharmaceuticals and life sciences with a volume of EUR500,000 to EUR100m. Dr. Storz organizes the annual Rhineland Biopatent Forum and regularly publishes articles on patent issues relating to therapeutic antibodies in the magazine mAbs. He is a member of the Antibody Society, the Licensing Executive Society (LES), the Association for the Protection of Intellectual Property (GRUR) and the Association of Intellectual Property Experts (VPP).
Abstract:
This presentation discusses the patent strategy underlying the world’s best selling drug, AbbVie’s Humira®. Despite a non-optimal starting position, AbbVie has established an extensive portfolio to fend off biosimilar competition. The presentation will discuss this portfolio in detail, and comment on its strengths and weaknesses.
Keynote Forum
Tsachi Shaked
E3D Elcam Drug Delivery Devices, Israel
Keynote: Innovative, cost effective solution for self-administration of bio-similar drugs
Time : 11:25-12:25

Biography:
Tsachi Shaked, MBA, is the Senior Director for Marketing and Business Development at E3D (Elcam Drug Delivery Devices) a subsidiary of Elcam Medical. He has done his Master’s in Business Administration (MBA) with major in Marketing from Bar-Ilan University in Ramat-Gan, Israel. He is involved in the development of the new version of drug delivery devices that includes connectivity and electronic applications. He has been working at E3D (Elcam Drug Delivery Devices) since 2006.
Abstract:
The purpose of this presentation is to identify and present a cost effective method, and devices for the self-administration of biosimilar drugs and molecules while keeping the entire process safe and easy to use. Disposable auto-injectors have their advantages of safe and simplicity, but pose an additional cost of materials to a bio-similar drug/molecule. Reusable auto injectors are more cost effective, but the ones in the market are complicated, are not easy to use and not completely safe. In this presentation we will present a new, innovative, method for easy and safe yet cost effective way for self-administration of biosimilar drugs/molecules. These innovative devices might be a perfect partner with the biosimilar drug as they are not only cost effective, safe and easy to use, but also have a lower environmental impact of plastic parts and trash. We will discuss mechanical auto-injectors (picture 1) and electronic auto-injectors (picture 2) while in both the only disposable part is the cassette that holds the PFS with the drug inside.
- Biosimilar Market and Cost Analysis | Analytical Strategies for Biosimilars | Globalization of Biosimilars
Location: Lilli Palmer
Chair
Tim Demuth
Sandoz, Germany
Session Introduction
Rosa Helena Bustos
University of La Sabana, Colombia
Title: CMC considerations for biosimilar drug development
Time : 13.25 - 14.05pm

Biography:
Bustos R H works on nanobiosensors development for evaluation and characterization of molecular interaction in biological drugs. She introduced the nanobiosensors (surface plasmon resonance and quartz crystal microbalance) as new research technology in Colombia. She is also the Group Leader of Therapeutic Evidence Group at Universidad de La Sabana. She and her group works on safety and efficacy in drugs, clinical pharmacology and pharmacovigilance. She has also participated in the elaboration of evaluation guidelines for biosimilars according to regulatory affair (INVIMA).
Abstract:
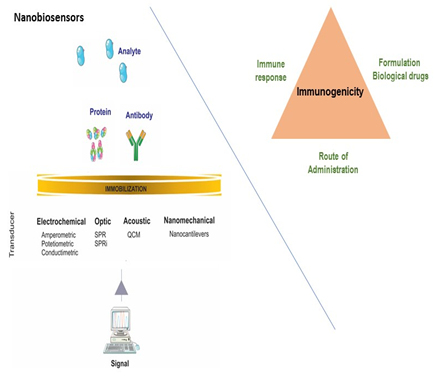
The scope of this speech is to show a general consideration regarding the evaluation of immunogenicity in bio-therapeutic products. Biological drugs and their biosimilars are highly complex molecules derived from living cells or organisms. The success of biological drugs has been focused on unwanted immunogenicity of these products. Evaluation of unwanted immunogenicity is an important consideration in development of bio-therapeutics products. Immunogenicity to protein based biological drugs is a complex process that involves numerous factors such as general patient´s immunity, amino-acid sequence of proteins, as well as biological drugs formulation (i.e. protein aggregates, impurities or excipients improper choice) and the drug administration. With new platforms, new technologies and development of novel therapeutics and treatment modalities, the assessment of immunogenicity will continue to require products approved on safety as well as on efficacy perspectives. Correlation between immunogenicity and clinical sequelae entirely depends on the appropriate detection, measurement and characterization of antibodies against biological therapeutics. These include conventional approaches such as ELISA and radioimmunoprecipitation assays. Alternative technologies use instruments based in nanobiosensors as surface plasmon resonance (SPR) and quartz crystal microbalances (QCM) to detect and characterize antibodies against therapeutic proteins and other molecular interactions. The purpose of this presentation is to show a general overview on the ability to detect therapeutic induced antibodies in plasma and sera of treated patients with biological drugs, using nanobiosensors. The evaluation, safety and efficacy of biosimilars require more rigorous testing than conventional generic drugs. The profiles of biosimilars must demonstrate similar physicochemical and biological characteristics, efficacy and safety in accordance with the approval requirements of regulatory authorities.
EfraÃn Esteban
University of La Sabana, Colombia
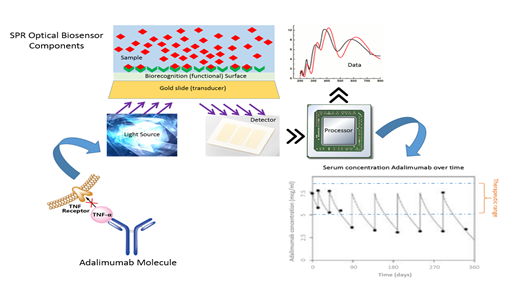
Title: Development of a methodology for the evaluation of tumor necrosis factor antibody (anti-TNF-alpha) in patients with treatment of biological products through the use of Nanobiosensors
Time : 14:05-14:45

Biography:
Efraín Esteban is a medical doctor interested in the evaluation of the relationship between serum levels and therapeutic efficacy in the use of biological drugs as well as the determination of immunogenicity in the clinical setting related to use of these molecules. Currently working in the Research Group Therapeutic Evidence of the Faculty of Medicine of the Universidad de La Sabana under the coordination of Dr. Rosa Bustos. In addition, they have participated in speeches and conferences regarding the safe and cost effective use of biological drugs in Colombia.
Abstract:
Rheumatoid Arthritis (RA) is an inflammatory disease of the joints that affects a large number of people worldwide. This condition can lead to debilitating joint destruction through the erosion of cartilage and bone. One of the pharmaceutical interventions for the treatment of this pathology are the biological disease-modifying anti-rheumatic drugs (DMARDs) that are directed toward inhibiting the cellular immune response responsible for the inflammation. TNF inhibitors were the first of the biological DMARDs to be approved for the treatment of RA and have become part of the routine treatment of patients with this disease. During the last years, the therapeutic drug monitoring of adalimumab and other biosimilar anti-TNF drugs have been an important tool to optimize the outcomes and costs of treatment, because the wide variations in their serum concentrations cannot be explained by classic pharmacokinetic factors and they are associated with therapeutic failures, anti-drug antibody formation, presence of adverse reactions, over dosage and in many cases treatment drop out. The methods for measurement of these drugs are expensive, with very high analysis time and for some molecules with low sensitivity and specificity. Biosensors is a nano-biosensor technology used for the study of interactions in real time, with advantages in the speed of analysis time, without the use of markers or developers and complying with the harmonization standards for biological analytical methodologies, allowing to customize the therapy and the use of concomitant immunomodulatory treatment. The objective of this study is to develop a real-time methodology through the use of an optic biosensor for the serum quantification of anti-TNF alpha in patients diagnosed with rheumatoid arthritis treated with anti-TNF alpha therapy.
Ulrich Storz
Michalski Hüttermann & Partner, Germany
Title: How AbbVie tries to fend off world’s blockbuster No 1 from generic competition

Biography:
Ulrich Storz received his PhD from the University of Münster in 2002 with a thesis in neurobiology. He then completed his training to become a German patent attorney. He joined the list of professional representatives before the European Patent Office in 2006. Dr. Storz's main areas of practice are managing and enforcing patents and patent applications as well as drafting FTO analyses and opinions. He also provides advice on strategic patent issues, notably in life sciences (biotechnology, biophysics, biochemistry and microbiology), above all in the field of therapeutic antibodies. Ulrich Storz is regularly involved in major antibody opposition cases before the European Patent Office. In recent years, he has also provided representation in a large number of sizeable due diligence projects in the field of pharmaceuticals and life sciences with a volume of EUR500,000 to EUR100m. Dr. Storz organizes the annual Rhineland Biopatent Forum and regularly publishes articles on patent issues relating to therapeutic antibodies in the magazine mAbs. He is a member of the Antibody Society, the Licensing Executive Society (LES), the Association for the Protection of Intellectual Property (GRUR) and the Association of Intellectual Property Experts (VPP).
Abstract:
This presentation discusses the patent strategy underlying the world’s best selling drug, AbbVie’s Humira®. Despite a non-optimal starting position, AbbVie has established an extensive portfolio to fend off biosimilar competition. The presentation will discuss this portfolio in detail, and comment on its strengths and weaknesses.
Tsachi Shaked
E3D Elcam Drug Delivery Devices, Israel
Title: Innovative, cost effective solution for self-administration of bio-similar drugs

Biography:
Tsachi Shaked, MBA, is the Senior Director for Marketing and Business Development at E3D (Elcam Drug Delivery Devices) a subsidiary of Elcam Medical. He has done his Master’s in Business Administration (MBA) with major in Marketing from Bar-Ilan University in Ramat-Gan, Israel. He is involved in the development of the new version of drug delivery devices that includes connectivity and electronic applications. He has been working at E3D (Elcam Drug Delivery Devices) since 2006.
Abstract:
The purpose of this presentation is to identify and present a cost effective method, and devices for the self-administration of biosimilar drugs and molecules while keeping the entire process safe and easy to use. Disposable auto-injectors have their advantages of safe and simplicity, but pose an additional cost of materials to a bio-similar drug/molecule. Reusable auto injectors are more cost effective, but the ones in the market are complicated, are not easy to use and not completely safe. In this presentation we will present a new, innovative, method for easy and safe yet cost effective way for self-administration of biosimilar drugs/molecules. These innovative devices might be a perfect partner with the biosimilar drug as they are not only cost effective, safe and easy to use, but also have a lower environmental impact of plastic parts and trash. We will discuss mechanical auto-injectors (picture 1) and electronic auto-injectors (picture 2) while in both the only disposable part is the cassette that holds the PFS with the drug inside.