Theme:
Biosimilars 2018

|
Conference Name |
Place |
Date |
|
Biosimilars 2018 |
Boston, MA, USA |
October 24-25, 2018 |
With less than 10 biosimilars approved by USFDA, the drug class is still in to infancy. However, market penetration rate is quite high with more biologic products going off patent. Amgen Inc.’s (AMGN) Amjevita, a biosimilar of AbbVie’s (ABBV) Humira (adalimumab) secured FDA approval in September 2016, but is yet to be marketed due to legal hurdles. Production bottlenecks are the second most obvious challenge since biosimilar manufacturing is complex, as the drugs are derived from living cells. This often leads to higher manufacturing costs and concerns about scalability potential of the manufactured biosimilars. Nevertheless, pricing is an important parameter requiring optimization. Pfizer Inc. (PFE) secured approval for Inflectra (infliximab-dyyb), its biosimilar of Johnson & Johnson’s (JNJ) Remicade, in April 2016. However, the price of Inflectra is only 15% lower than that of Remicade, and the much-anticipated launch dented the hopes of deep discounts from biosims. Generics usually cost 80% less than branded drugs.
With so many challenges in research, still class of drug is experiencing the maximum funding with great Returns on Investment (ROI). Keeping these points in mind we are organizing the 13th International Conference on Biologics and Biosimilars during October 24-25, 2018 at Boston, USA with the theme: Emerging Trends in Biosimilars Development and Approval.
Why to attend Biosimilars 2018:
Who do you meet at Biosimilars 2018
Track 1: Current Challenges in Developing Biosimilars

The development of biologics calls for overcoming many challenges. With initial steps of concepts of biologics, their considerations, essentials for early clinical developments it is very much needed that proper scientific and strategic approaches are taken for the successful development of follow-on-biologics. Moreover, the need for overcoming the challenges continues in the late clinical steps, drug safety factors and labeling requirements. Also it is much required now to develop a drug product in accordance to Quality by Design (QbD).
This biosimilars conference will look at the facets of current challenges in biosimilar development. This biosimilar conference will focus on multiple aspects of biosimilar product development to successfully deliver safe, potential and efficacious biologic products to the market.
Track 2: Biopharmaceuticals
A biopharmaceutical is also known as a biologic(al) medical product. It is any pharmaceutical drug product which is manufactured in, extracted from, or semisynthesized from biological sources. They are different from totally synthesized pharmaceuticals. They include vaccines, blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living cells used in cell therapy.
Track 3: Biosimilars Analytical Strategies

Analysis of biosimilars and biologics forms to be one of the most important aspect towards the biologics and biosimilar development process. Biosimilars analytical methods for process development and validation as well as use of production technologies such as disposables and supply chain logistics can help companies establish facility flexibility.
This biosimilars global event also includes Bioanalytical methods, Formulation, Bioassay for comparability and potency testing, GMP protein analysis, LC/MS analysis for discovery, preclinical, and clinical programs.
Track 4: Emerging Biosimilars in Therapeutics

The explorations in the field of biologics have created a new avenue for the clinicians towards better disease management. The emerging biologics have already manifested fruitful outcomes in treatment of ailments like those of psoriasis, rheumatic arthritis, certain cancers, inflammatory bowel disease (IBD) etc. Emerging Biosimilar insulins are likely to enter the insulin landscape as patents for major branded insulin products start to expire in the next few years. The main theme of this track is to have sound knowledge in the emerging biosimilars like Filgrastim, Pegfilgrastim, Recombinant blood products, therapeutic proteins, vaccines, Biosimilar anti-bodies, Growth hormones, Biosimilar peptides, therapeutic proteins and other biosimilar developments and their impacts.
Track 5: Regulatory Approach for Biosimilars

Biosimilars are the generic version of biological. It is the new buzz word in pharmaceutical industry. Biosimilars are highly similar to licensed reference product not withstanding minor differences in clinically inactive components; also there are no clinically meaningful differences between the biologicals and the reference product in terms of safety, purity, and potency. This track includes: Licensing of biosimilars, Biosimilars regulation, Patent issues, BLA filing for biosimilars, Biosimilars regulatory prospects of BRIC countries, a paradigm of traditional generics to Biosimilars , Biowaiver approval for Biosimilars and other aspects of Biosimilar approvals. Biosimilars 2017 will provide an excellent and global opportunity to the scientists, partners and pharma leaders from Biopharmaceutical and Biotechnology industries to innovate and to explore the strategic market for Biosimilars and Biologics with a clear picture of the regulatory approach for biosimilars and biologics.
Track 6: Legal Issues and BPCI Act

The Patient Protection and Affordable Care Act (PPAC Act) was signed into law in March 2010 in addition to the amendments in the Public Health Service Act (PHS Act) to create an abbreviated approval pathway for biosimilars and follow on biologics. These new statutory provisions are often referred as the Biologics Price Competition and Innovation Act of 2009 (BPCI Act). This track concentrates upon such legal bindings and the aspects of the BPCI Act that pertain to the biosimilars and biologics. This session on legal issues shall be very beneficial to research scientists from both academic backgrounds and also those from industry R&D.
Track 7: Biosimilars Research Pipeline

Biosimilars is a biologic medical product which is copy of an original product that is manufactured by a different company. There are some specific scientific consideration for criteria, design and analysis regarding development of Biosimilars. Biosimilars are officially approved versions of original biosimilar innovator products, and can be manufactured when the original product's patent expires.
This session shall be highly beneficial for the biosimilar industry researchers to update themselves on the latest research updates from around the world. This session also finds place for all the biosimilar exhibitions associated with the field of biosimilars and biologics.
Track 8: Globalization of Biosimilars

This track discuses about the generic drugs impact on global biosimilar market , Cost and risk management, Adopting innovative mechanisms such as risk-sharing arrangement, European market for biosimilars.
The global market scenario with the launch of first biosimilar in the market forecasts some radical changes. This track will look upon such key concerns which are witnessed by the global pharma market and that are coming up with the subsequent launch of the other biosimilars and biologics. Despite these emerging facilities, biotherapeutic developers are most comfortable off-shoring to established markets—the US and Europe.
Track 9: Drug Delivery and Development
Drug Delivery Companies and Market session is beginning to change for small, medium, and large scale pharmaceutical Companies. Biopharmaceutical Manufacturer and Industries and generic drugs companies contract drug delivery companies which can manifest from development to manufacturing. Addressing these instabilities is a great challenge, because of the complexity of the Clinical bio therapeutics themselves.
Track 10: Clinical Development Of Biosimilars

This track includes Clinical trials on major diseases Risk management, and quality affairs, Case studies, and clinical models, Transgenic animals, Targeted cell line development, PK/PD studies, Toxicological studies, ethics maintained in clinical and preclinical studies, development difficulties and Aspects of genotoxicity tests. Biosimilar guidelines on the above mentioned topics are also to be thrown light upon at this biosimilars conference.
Track 11: Intellectual Property Rights

The safeguarding of product trade secret, its formulations and other process parameters by law is usually covered by IPR. It includes those as patents, copyrights, industrial design rights, trademarks etc. IPR is of prime importance in the field of biologics and biosimilars. Most scientist and industries tend to retain their monopoly business by exercising the IPR.
The very name Biosimilars calls for the occurrence of Intellectual Property rights laws and by-laws. Hence this session is of utmost interest to the attorneys and law personnel.
Track 12: Current Trends in Pharmaceutical industry

This track covers all the current trends that are coming up in the Pharmaceutical industries to provide better pharmaceutical products.
Track 13: Bioequivalence Assessment

Bioequivalence focuses on the equivalence of release of the active pharmaceutical ingredient from the pharmaceutical product and its subsequent absorption into the systemic circulation. This session has utmost importance in context to the fact that only a suitably bioequivalent drug candidate that conforms the results in all respects to the original licensed product can be called as biosimilar drug.
Of all attempts towards developing a follow on biologics or a biosimilar drug the main detection point stands at the bioequivalence assessment. Once the bioequivalence has been obtained it can be 70% ascertained the drug qualifies to be a suitable biologics or biosimilars.
Track 14: BCS and IVIVC Based Biowaivers

The objective of this work was to suggest the biowaivers potential of biopharmaceutical classification system which is known to increase the solubility, dissolution, oral absorption of water insoluble drugs. Biopharmaceutics Classification System and invitro and invivo classification discusses about ADME pathways of different drugs. This also includes BCS biowaivers, In vitro diffusion cells for dissolution testing in formulation development, In vitro preclinical ADME/BCS testing.
Track 15: Biosimilar Companies and Market Analysis
This track is concentrated towards the different reviews and forecasts regarding the scenario of Biosimilars market and follow on Biologics. The present status and future scenario of the market are best to be discussed during this session. Market researches from the first launching of biosimilar to the newest one till date prospects for a radical change in the pharmaceutical market.
Track 16: Challenges in Biosimilars Pharmacovigilance

This session of the Biosimilars 2018 looks into the future and FDA initiatives that have already been announced to include enhanced tracking and follow-up of post market issues, planned improvements in AERS, and pilots of new post market drug-monitoring strategies. Current challenges in pharmacovigilance, Adverse drug reactions with pharmaceutical products, Biosimilar guidelines for pharmacovigilance practice and pharmacoepidemiology are the points that shall be laid emphasis in this session.
Track 17: Brexit Effect on Biosimilars

The Brexit effect on Biosimilars tends to be negative. Not only would it be a major setback towards approval and launch of biosimilars to the market but also it would be hindrance towards the cost cutting approach taken up by NHS. With Britain being among principal clinical trial centers is owned to see a decrease in the willingness of the manufacturers and researchers to carry out any further trials in Britain. Also Brexit will cause the principal motive of British Biosimilars Association (BBA) to fall back- which aimed at increasing the use of biosimilars.
Track 18: Entrepreneurs Investment Meet

Entrepreneurs who are willing to put in hard work and invest in the field of biologics and biosimilars will find this meeting the best place to properly shape their drive for the new endeavors. Also this meeting will help them find the best experts who can make their investment fruitful and worthwhile.
Biosimilars adoption in developed countries has been mostly payer-driven , based upon payers’ urgency and unmet need to contain public health care expenditures. In today’s emerging markets, biosimilars are still in a status that is nascent, with little to no marked presence. A recent Kantor Health Survey found that 20 percent of emerging market autoimmune patients use a biologic, with the distribution of biologics varying from 29 percent in China to 12 percent in Russia and a mere 6 percent in Brazil.
Biosimilars in USA:
- USFDA approved the first biosimilar in March 2015 with Sandoz’s Zarxio (filgrastim)
- Close to 19 pipeline biosimilar molecules are in development phase
- Bisoimilars represent about 50% of the global biologics market value and generates about 50% of the sales value growth
- Further approvals would be based on pending legislative decisions on data exclusivity period, naming conventions and interchangeability
Biosimilars in European Union:
- By far the largest biosimilar market representing 80% of global biosimilar spending
- Nineteen biosimilar products authorized in four molecule classes: human growth hormone, erythropoietin, G-CSF and tumour necrosis factor (TNF)-inhibitor
- About 29 pipeline biosimilars molecules in development
- Most organized and unambiguous approval pathway
- Pharmacy level substitution prominently into practice
- Payer-driven uptake
-
Biosimilars are an integral part of the effective biological therapies available in the EU
Biosimilars in Asia:
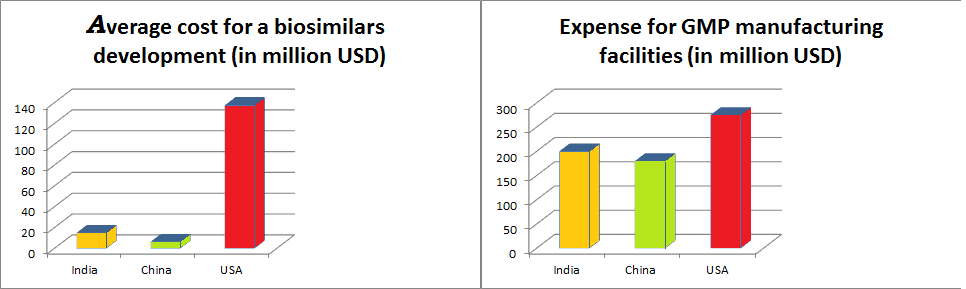
- Asia’s infrastructural availability can vouch for lower biosimilar development costs (e.g., US$11M–$20M in India and US$3M–$10M in China, versus US$100M–$250M in the U.S. and developed countries)
- Asia countries will require Lower capital expenditure for GMP manufacturing facilities (US$250–$300M in the U.S.; 25–50% cheaper in Japan, India, South Korea and China)
- Incur Lower labor costs in Asia (4%–73% of the cost of U.S. labor)
- To date, China and India have approved 96 and 66 follow-on biologics respectively, while Japan and South Korea have approved 9 and 7 biosimilars, respectively
- China has also provided generous funding, with US$308B allocated from 2010 to 2015 for biotechnology including biosimilars, and an additional US$11.8B earmarked for 2015-2020 to advance innovations in biotech.
|
|
Access to Biologic products |
Regulatory Stringency |
Payer's Perspectives |
Prescriber's Perspective |
Patient Acceptance |
Available Biosimilars in market |
||||||||
|
|
|
|
|
|
0-5 | ||||||||
|
|
|
|
|
|
>12 | ||||||||
| Japan | High |
|
|
|
|
~10 | ||||||||
|
|
|
|
|
|
<5 | ||||||||
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
||||||||
| Brazil | Low |
|
|
|
|
|
||||||||
| Russia |
|
|
Low |
|
|
<5 | ||||||||
| India | Low |
|
|
|
|
>12 | ||||||||
| China | Low |
|
|
|
|
~5 | ||||||||
| South Africa | Low |
|
|
High | Moderate |
<5 |
Biosimilars 2018
Conference Series hosted the 11th European Biosimilars Congress at Holiday Inn Rome Aurelia via Aurelia, Km 8.400 00165 Rome, Italy during April 26-27, 2018. The conference was designed around the theme of “The developmental strategies and uptake of biosimilars through a decade in Europe” and was a great success where eminent keynote speakers from various reputed companies made their resplendent presence and addressed the gathering. Moreover, the networking sessions laid the foundation for some time worthy collaborations between many start-up and big industries. The post conference networking lunch session witnessed a number of B2B meetings that are turning up to be mutually beneficial to both the organizations who had gone in for the business meetings.
Euro Biosimilars 2018 witnessed an amalgamation of peerless speakers who enlightened the crowd with their knowledge and confabulated on various new-fangled topics related to the field of Biologics and Biosimilars. This congress not only brought forward the latest developments in the field but also provided solutions to the numerous challenges encountered in developing a biosimilars.
Conference Series would like to convey a warm gratitude to all the Honorable guests, Keynote Speakers, Delegates, Media Partners and Exhibitors for their participation in Euro Biosimilars 2018.
Andras Guttman, SCIEX, USA
Fiona M Greer, SGS Life Sciences, Switzerland
David Ebsworth, Vifor Pharma Ltd, Switzerland
Parastoo Azadi, Complex Carbohydrtae Research Center - University of Georgia, USA
Cecil Nick, PAREXEL International, UK
Dominic Seitz, Simon-Kucher & Partners, Germany
Sakae Tsuda, National Institute of Advanced Industrial Science and Technology, Japan
Kamali Chance, BioSciencesCorp, USA
Roberta Bursi, InsilicoTRIALS, Italy
Oleksandr Kukharchuk, ReeLabs Pvt. Ltd., India
We on behalf of the conference specially thank the exhibitors for their participation in the congress and also the media partners for their wonderful marketing of the event. Conference Series also took the privilege of felicitating Euro Biosimilars 2018 Organizing Committee, Keynote Speakers and Chair whose support made conference a great success.
With the enormous feedback from the participants and supporters of Euro Biosimilars 2018, we are glad to announce 12th European Biosimilars Congress during April 15-16, 2019 at Berlin, Germany.
Biosimilars 2017
Conference Series hosted the 7th European Biosimilars Congress at Novotel München City Arnulfpark Munich, Germany during May 15-16, 2017.The conference was designed around the theme of “The developmental strategies and uptake of biosimilars through a decade in Europe” and was a great success where eminent keynote speakers from various reputed companies made their resplendent presence and addressed the gathering. Moreover, the networking sessions laid the foundation for some time worthy collaborations between many start-up and big industries. The post conference networking lunch session witnessed a number of B2B meetings that are turning up to be mutually beneficial to both the organizations who had gone in for the business meetings.
Euro Biosimilars 2017 witnessed an amalgamation of peerless speakers who enlightened the crowd with their knowledge and confabulated on various new-fangled topics related to the field of Biologics and Biosimilars. This congress not only brought forward the latest developments in the field but also provided solutions to the numerous challenges encountered in developing a biosimilar.
Conference Series would like to convey a warm gratitude to all the Honorable guests, Keynote Speakers, Delegates, Media Partners and Exhibitors for their participation in Euro Biosimilars 2017.
Christoph Volpers, Michalski & Hüttermann, Germany
Arthur G Cook, ZS Associates, USA
Tim Demuth, Sandoz Pharmaceuticals GmbH, Germany
Rosa Helena Bustos, University of La Sabana, Colombia
Ulrike Konrad, Protagen Protein Services , Germany
Stanley Seung Suh Hong, Celltrion Healthcare Co. Ltd., South Korea
Ulrich Storz, Michalski Hüttermann & Partner, Germany
We on behalf of the conference specially thank the exhibitors for their participation in the congress and also the media partners for their wonderful marketing of the event. Conference Series also took the privilege of felicitating Euro Biosimilars 2017 Organizing Committee, Keynote Speakers and Chair whose support made conference a great success.
With the enormous feedback from the participants and supporters of Euro Biosimilars 2017, we are glad to announce 11th European Biosimilars Congress during April 26-27, 2018 Rome, Italy.
Euro Biosimilars 2016
Conference Series hosted the 5th European Biosimilars Congress at Hotel Meliá Valencia in Valencia, Spain during June 27-29, 2016.The conference was designed around the theme of “The European perspective of Biologics and Biosimilars” and was a great success where eminent keynote speakers from various reputed companies made their resplendent presence and addressed the gathering. Moreover, the networking sessions laid the foundation for some time worthy collaborations between many start-up and big industries. The post conference networking lunch session witnessed a number of B2B meetings that are turning up to be mutually beneficial to both the organizations who had gone in for the business meetings.
Euro Biosimilars 2016 witnessed an amalgamation of peerless speakers who enlightened the crowd with their knowledge and confabulated on various new-fangled topics related to the field of Biologics and Biosimilars. This congress not only brought forward the latest developments in the field but also provided solutions to the numerous challenges encountered in developing a biosimilar.
Conference Series would like to convey a warm gratitude to all the Honorable guests, Keynote Speakers, Delegates, Media Partners and Exhibitors for their participation in Euro Biosimilars 2016.
Laszlo Endrenyi, University of Toronto, Canada
Candida Fratazzi, BBCR Consulting, USA
Kamali Chance, Quintiles Inc., USA
Andreu Soldevila, Lean Biopro, Spain
Ruideger Janwkosky, Cinfa Biotech GmbH, Germany
We on behalf of the conference specially thank the exhibitors for their participation in the congress and also the media partners for their wonderful marketing of the event. Conference Series LLC also took the privilege of felicitating Euro Biosimilars 2016 Organizing Committee, Keynote Speakers, Chair and Co-Chairs whose support made conference a great success.
With the enormous feedback from the participants and supporters of Euro Biosimilars 2016, we are glad to announce 7th European Biosimilars Congress during May 15-17, 2017 at Munich, Germany and also invite you to participate at our 6th International Conference and Exhibition on Biologics and Biosimilars through October 19-21, 2016 at Houston, TX, USA.
Touro College of Pharmacy organized a one day symposium cum pre-conference workshop on March 29, 2016 with a focus on “Advances in Generic Pharmaceuticals and Biosimilars”. The symposium was held at the Manhattan Campus of the Touro College of Pharmacy located at 231 West 124th Street. Leading scientists in the pharmaceutical and biotechnology industry as well as in academia were present seminars addressing advances in the development, manufacturing, marketing and regulatory pathways of generics and biosimilars. The symposium was sponsored by ConferenceSeries Ltd.
The keynote speeches of the renowned industry professionals and academicians was a real value addition to all the attendees. It was a perfect platform for exchange of scientific knowledge between the industry and academic sector. A well organized pre conference workshop was conducted and we look forward towards the attendees to attend the Biosimilars 2016 conference in Houston, TX, USA during October 19-21, 2016.
Conference Highlights
- Current Challenges in Developing Biosimilars
- Emerging Biosimilars in Therapeutics
- Biosimilars Analytical Strategies
- Regulatory Approach for Biosimilars
- Legal Issues and BPCI Act
- Biosimilars Research Pipeline
- Globalization of Biosimilars
- Clinical Development Of Biosimilars
- Intellectual Property Rights
- Bioequivalence Assessment
- BCS and IVIVC Based Biowaivers
- Biosimilar Companies and Market Analysis
- Challenges in Biosimilars Pharmacovigilance
- Brexit Effect on Biosimilars
- Entrepreneurs Investment Meet
- Biopharmaceuticals
- Drug Delivery and Development
- Current Trends in Pharmaceutical industry
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | October 24-25, 2018 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Bioanalysis & Biomedicine
- Journal of Bioequivalence & Bioavailability
- Journal of Pharmaceutical Sciences & Emerging Drugs
Abstracts will be provided with Digital Object Identifier by



























































































