Dina E Abo Elezz
Pharos University, Egypt
Title: Development and in-vivo evaluation of Ofloxacin gastro-retentive drug delivery system
Biography
Biography: Dina E Abo Elezz
Abstract
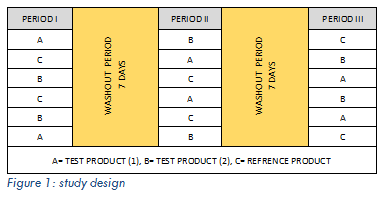
Ofloxacin is readily soluble in the acidic environment of the stomach, following oral administration; there is rapid and extensive oral absorption from the gastrointestinal tract. Gastric retention is designed to prolong gastric residence time of oral controlled release dosage forms which enhance therapeutic efficacy, reduce time intervals for drug administration, potentially reduced dose size and thus improved patient compliance. Factorial designs of 23 were planned for the optimization of ofloxacin floating and size increasing tablets, using Design-Expert® software. Convenient parameters which measures the floating lag time, floating duration, size increasing behavior, t50% and drug release tests were performed in 0.1 N HCl at 37ºC of the suggested formulations of the design, where different concentrations of different polymers (HPMC K100M, HPMC K15M and PEO wsr303) were used. Responses of the floating preparation were principally related to the polymer type rather than polymer concentration, while responses of the size increasing preparation were related to both polymer type and concentration. Comparative randomized, single dose, three-way crossover open label study was done to evaluate in vivo release and pharmacokinetics parameters of two different ofloxacin gastroretentive tablets 400 mg/tablet (test product 1, 2) relative to Tarivid® conventional tablet (2x200 mg/tablet) (reference product) after single oral administration to six healthy adult males as shown in Figure 1. Drugs were administered to fasted volunteers and blood samples were collected up to 24 hours and assayed for ofloxacin using a validated LC-MS/MS method. The pharmacokinetic parameters AUC0-t, AUC0-∞, Cmax, Tmax, T1/2, MRT and elimination rate constant were determined from plasma concentration-time profile by non-compartmental analysis method.