
Abdel Qader Al Bawab
Al-Zaytoonah University of Jordan, Jordon
Title: Comparative randomized, single dose, two-way crossover open label study to determine the bioequivalence of two formulations of Alfuzosin tablets
Biography
Abdel Qader Al Bawab is a Pharmacist from Jordan. He has completed his Master’s degree in Biopharmaceutical from University of New South Wales, Australia in 2006 and in 2012 he attained a PhD degree in Clinical Pharmacy from Queens’s University Belfast. He is interested in clinical pharmacokinetics, population pharmacokinetic and bioequivalence studies.
Abstract
Alfuzosin is an α1 blocking medication that is approved by the US FDA to treat Benign Prostate Hyperplasia (BPH) by relaxing the muscles in the prostate and bladder neck, making it easier to urinate. The branded alfuzosin is an expensive option; hence, the availability of generic alfuzosin will provide better access to the medication, especially for non-insured patients with BPH. Bioequivalence studies are demanded by the regulatory authorities to allow the marketing of new generics of alfuzosin. The aim of this study is to assess the bioavailability of the generic (test) and branded (reference) formulations of 10 mg alfuzosin of extended release (XR) tablets after oral administration to healthy adults under fed conditions. The current report methodology was based on a comparative, randomized, single dose, two-way crossover open label study design. Thirty three subjects were given a single dose of the test alfuzosin tablet and completed the clinical study. The pharmacokinetic parameters Cmax and AUC0→t, Kel, AUC0→∞, tmax, t1/2el were estimated to prove bioequivalence. The confidence intervals for the log-transformed Test/Reference Ratios for alfuzosin 110.65 (98.01-124.93) % and 111.98 (101.87-123.10) % for Cmax and AUC0→∞, respectively, were within the allowed limit specified by the regulatory authorities (75-133% for Cmax and 80-125% for AUC0→∞). Hence, clinically, the test tablet can be prescribed as an alternative to the reference for the indication of trewting patients with BPH.
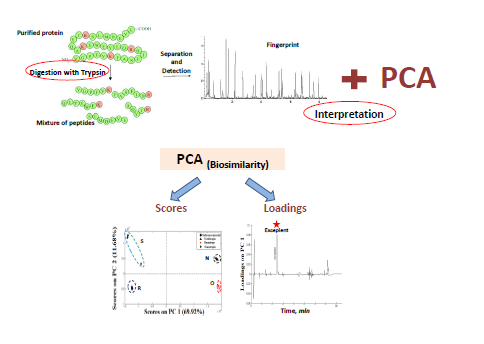
Basma M Eltanany
Cairo University, Egypt
Title: Chemometrics in biosimilarity and stability assessment; Analytical review
Biography
Basma M Eltanany is an Assistant Professor of Analytical Chemistry and Instrumental Analysis in the Faculty of Pharmacy at Cairo University, Egypt. She is a Postdoctoral Researcher at the same faculty. She has completed her BSc in Pharmaceutical Sciences and her MSc in Analytical Chemistry in 2006 and 2012, respectively and a PhD degree in Analytical Chemistry in 2016. She has published number of articles in referred journals and participated in national and international conferences. She was selected and awarded a scholarship for academic staff mobility (TEACHING AND TRAINING) at University of Porto, Portugal. Her current research interests include biosimilarity assessment, Chemometrics and separation sciences. Her interests also include development and validation of analytical and bioanalytical methods for determination of pharmaceutical compounds in different matrices.
Abstract
Statement of the Problem: Nowadays, biological is not a future dreams, biological is a fundamental part of healthcare worldwide with growing needs to safe, efficient, interchangeable and cost effective biosimilars. At this end, simple, accurate and reliable biopharmaceutical analysis is a great priority for both industry and regulatory authorities. Aim: The aim of this work was to review, discuss and focus on statistically guided approaches used along with many analytical techniques for biosimilarity and stability assessment. Methodology & Theoretical Orientation: Principal component analysis was the main unsupervised chemometric model to be highlighted. Other models coupled to different analytical techniques (NMR, HPLC-UV and HPLC-MS/MS) were also reviewed. Findings: This coupling was found to be competent for detecting the similarities and dissimilarities between samples and also determining to what extent different samples are actually "different". Conclusion & Significance: The mathematical modeling of big multivariate analytical data has given very informative and rewarding outcome that could be beneficial to National Control Laboratories especially in countries with price sensitive markets where the exhaustive assessment of imported biotechnological products including biosimilars is crucial. Recommendations are made for inclusion of chemometrics laboratory at each organization committed with research and control of biologics. Recent Publications 1.Sara M Shatat, Basma M Eltanany, Abeer A Mohamed, Medhat A Al-Ghobashy, Faten A Fathallaa and Samah S Abbas (2018) Coupling of on-column trypsin digestion–peptide mapping and principal component analysis for stability and biosimilarity assessment of recombinant human growth hormone. Journal of Chromatography B 1072:105-115. 2.Jawed Fareed, Peter Bacher and Walter Jeske (2018) Advances in heparins and related research: an epilogue. Molecules 23(2). 3.Kang Chen, Junyong Park, Feng Li, Sharadrao M Patil and David A Keire (2017) Chemometric methods to quantify 1D and 2D NMR spectral differences among similar protein therapeutics. AAPS PharmSciTech 19(3):1011-1019. 4. BoÅ¡tjan Japelj, Gregor Ilc, Jaka MaruÅ¡iÄ, Jure SenÄar, Drago Kuzman and Janez Plavec (2016) Biosimilar structural comparability assessment by NMR: from small proteins to monoclonal antibodies. Scientific Reports
