Theme:
European Biosimilars Congress 2019

Welcome Message
by
Dr. Fiona Greer
Global Director
12th European Biosimilars Congress organized by Conference Series, scheduled April 15-16, 2019 Berlin, Germany.
I am delighted to learn that Conference Series is organizing the 12th European Biosimilars Congress, in Berlin, Germany, during April 15-16, 2019. The theme is “Development Strategies and Uptake of Biosimilars through a Decade in Europe”
In Europe we have seen the evolution of a science-based regulatory framework which has allowed over 40 biosimilar medicines to come to market within the EU, driven development of biosimilar regulatory guidelines internationally and transformed healthcare in many countries.
The 2019 conference will focus on three main tracks - key areas for biosimilar development: (1) Current Challenges in Developing Biosimilars, (2) Analytical Strategies for Biosimilars and (3) Biopharmaceutical Informatics. Presentations will include topics on manufacturing, analytical characterization, pre-clinical, clinical, regulatory strategies, global commercial challenges and market access strategies.
I believe that during the two days of conference, new knowledge and ideas will be presented, exchanged and actively discussed between participants from diverse backgrounds.
On behalf of the organizing committee, it gives me great pleasure to welcome all participants, both experts and those entering the field, to enjoy a rich, varied and attractive scientific and cultural program.
Your presence and deliberation will make this congress remarkably successful in all aspects of Biosimilars.
Fiona Greer
Global Director, Biopharmaceutical Services;
SGS, Switzerland
With 10 years now added to its repertoire, European Biosimilars Congress is really turning into a staple meeting where Biosimilars partners accumulate to address the present and future condition of Biosimilars in the Europe.
The expansion of two devoted streams for the European Biosimilars Congress meeting was a hit – we could pick up knowledge from two particular groups – one that exceeded expectations in the systematic and assembling end – and one that exceeded expectations in the business and key end.
The 2019 establishment of this meeting will enable us to keep on diving further into both the investigation of Biosimilars improvement – and the business requirements for organizations that keep on seeking FDA endorsement.
The Organizing Committee is delighted to invite you to attend the 12th European Biosimilars Congress one of its remarkable Pharmaceutical conferences, to be held during April 15-16, 2019 in Berlin, Germany. European Biosimilars Congress is a global annual event. This European Biosimilars Congress 2019 will bring together scientists, researchers, business development managers, CEOs, directors, IP Attorneys, Regulatory Officials and CROs from around the world. Many biologics products are making their entry in the pharma market and experiencing a notable rise in their usage over the conventional medications.
At Euro Biosimilars 2019 meet your target audiences from around the world focused on learning about biologics and Biosimilars. This conference would be your single best opportunity to reach the largest assemblage of participants from the biologics and Biosimilars community.
2019 Highlights:
- 300+ Participation (70 Industry: 30 Academia)
- 10+ Keynote Speakers
- 50+ Plenary Speakers
- 20+ Exhibitors
- 14 Innovative Educational Sessions
- 5+ Workshops
- B2B Meetings
Motives to attend:
- Keynote presentation along with interactions to galvanize the scientific community.
- Workshop and symposiums to reach the largest assemblage of participants from the Pharma/Biotech community.
- A wide track of exhibitors to showcase the new and emerging technologies.
- Platform to global investment community to connect with stakeholders in Pharma/Biotech sector.
- Young Scientist/ Investigators Award geared towards best budding young research.
- Links to the political marketing resources in order to expand your business and research network.
- Triumph of Awards, Certificates recognizes your commitment to your profession to encourage the nascent research.
Euro Biosimilars 2019 has everything you need:
Open panel discussions: Providing an open forum with experts from academia and business to discuss on current challenges in Biosimilars & Biologics, where all attendees can interact with the panel followed by a Q&A session.
Speaker and poster presentations: Providing a platform to all academicians and industry professionals to share their research thoughts and findings through a speech or a poster presentation.
Editorial board meeting: Discussing on growth and development of open access Bioanalysis and Biomedicine International Journals and recruiting board members and reviewers who can support the journal.
Round table meetings: Providing a platform where industry professionals meet academic experts.
Over 50+ organizations and international pavilions will be exhibiting at the Euro Biosimilars 2019 conference. . Exhibitors will include equipment manufacturers and suppliers, systems providers, finance and investment firms, R&D companies, project developers, trade associations, and government agencies.
In addition to the products and services you will have access to valuable content, including Keynote Presentations, Product Demonstrations and Educational Sessions from today’s industry leaders.
The Euro Biosimilars 2019 has everything you need, all under one roof, saving you both time and money. It is the event you cannot afford to miss!
Target Audience:
- Directors, CEO’s of Organizations
- Business Development Managers
- Chief Scientific Officers
- R&D Researchers from Biosimilar and Bioslogics Industries
- Professors, Associate Professors, Assistant Professors
- PhD Scholars
- Patent Attorneys
- Intellectual Property Attorneys
- Investment Analysts
- Association, Association presidents and professionals
- Noble laureates in Health Care and Medicine
- Bio instruments Professionals
- Bio-informatics Professionals
- Software development companies
- Research Institutes and members
- Supply Chain companies
- Manufacturing Companies
- CRO and DATA management Companies
- Training Institutes
- Business Entrepreneurs
Track 1: Current Challenges in Developing Biosimilars
The development of biologics calls for overcoming lot many challenges. With initial steps of concepts of biologics, their considerations, essentials for early clinical developments it is very much needed that proper scientific and strategic approaches are taken for the successful development of follow-on-biologics. Moreover, the need for overcoming the challenges continues in the late clinical steps, drug safety factors and labelling requirements. Also it is much required now to develop a drug product in accordance to quality by design (QbD).This Eurobiosimilars conference will look at the multiple facets of current challenges in Biosimilars development. This biosimilars conference will focus on multiple aspects of biosimilars product development to successfully deliver safe, potential and efficacious biologic products to the market.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailablity, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 2: Chemical and Analytical Strategies for Biosimilars
Management of cGMP facility calls for a strict monitoring all factors including analytical strategies, formulation procedures, packaging etc. For biologic products establishing comparability and interchangeability is a big hurdle. For this purpose employment of suitable analytical approach, bioassay, protein analysis, potency testing, safety assurances are highly important. LC/MS analysis for biologic products, characterization of biologics, peptide mapping, Isoelectric Focusing and Capillary Isoelectric Focusing, SDS-PAGE, Thermal Analysis, Particulate Matter Analysis, Thermogravimetric Analysis are some methods commonly used for analysis of biologics and Biosimilars products. On average, facilities outsource 32% of their analytical testing/bioassays (up from 28%) meaning that close to one-third of analytical testing is estimated to be outsourced by the industry.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailablity, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 3: Intellectual Property Rights
The safeguarding of product trade secret, its formulations and other process parameters by law is usually covered by IPR. It includes those as patents, copyrights, industrial design rights, trademarks etc. IPR is of prime importance in the field of biologics and biosimilars. Most scientist and industries tend to retain their monopoly business by exercising the IPR. The very name Biosimilars calls for the occurrence of Intellectual Property rights laws and by-laws. Hence this session is of utmost interest to the attorneys and law personnel. Currently, the US provides 12 years of exclusivity for new biological products under the Biologics Price Competition and Innovation Act (BPCIA).The provision providing 12 years exclusivity was buried inside the 20,000-page healthcare law. Eight years of exclusivity would keep biologic medicines out of the hands of many who need them. Prices frequently exceed $100,000.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 4: Biological Medicine
Biological Medicine works with the biology of the body and its natural healing capabilities as well as the spiritual, emotional and physical aspects of disease. Disease means that the body’s regulation is not working properly and needs to be brought back into its natural dynamic state where the immune system is in full regulation. It therefore looks for root causes for the presenting symptoms of disease the underlying factors causing a person to present with a certain illness. These root causes may consist of several factors which have built up over time and can include; diet, food allergies, intestinal disturbances, family history, stress, environmental factors, heavy metals, dental problems, hyperacidity, trauma, exposure to bacteria or viruses or electromagnetic disturbances.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 5: Emerging Biosimilars in Therapeutics
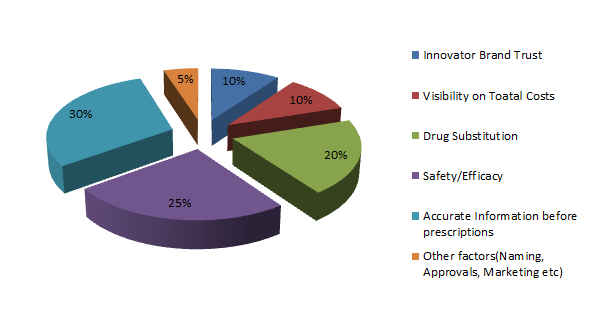
Biosimilars Market is experiencing a growth at an exponential rate. Presently around 700 biologics are making progress in the research pipelines of nearly 250 biopharma companies. Biosimilar insulins have already started revolutionizing the future drug development in the realm of diabetology. Biosimilars of Adalimumab, Etanercept, Rituximab, Peg-Filgrastim, Trastuzumab are expected to hit the market soon. Biosimilar of Humatrope, biosimilar of Eprex, biosimilar of Neupogen, biosimilar of Remicade have already been enjoying a greater market share in Europe than the reference product itself. The proportion of different biosimilars that reached market are Low Molecular Weight Heparins 44%, Epoetins 19%, HGH 11%, G-CSFs 7%, Interferons 6%, Insulins 5%, Others 8%.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailablity, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 6: Innovative Clinical Approach in Biosimilars
Clinical trials for biosimilars must exhibit practically identical security what's more, viability to the reference item, including consecutive PK/ PD and viability/security trials. Controllers expect PK/PD similarity information from a Phase I trial will bolster encourage viability/ wellbeing evaluations in crucial Phase III trials. Remain solitary Phase III investigations or joined Phase I/III outlines without supporting PK information are probably not going to be acknowledged. Clinical similarity prerequisites may fluctuate on a case-by-case premise subject to a risk based approach. Three-arm Phase I trials are progressively being used to show equivalence between the biosimilars and two authorized adaptations of a similar reference item that may exist in various markets, allowing developers to proceed with pivotal trials using a single version of the reference product
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 7: Regulatory updates on Biosimilars
Competitions and/or success in the present pharma industry are determined by the winning patent strategy which mostly pertains to the generic market entry. Generic and branded drug manufacturers both the patent strategy proximally belongs to the Hatch-Waxman Act statutory scheme and ANDA litigations. The Hatch-Waxman Act enacted in 1984 with amendment in 2003 facilitated the entry of generics at an early stage-thereby finishing the battle of branded generic ANDA of blockbuster drugs. All the same the Biologics Price Competition and Innovation(BPCI) Act has maximized the branded-generic patent duel in the biologics realm by imposing a litigated framework on follow-on-biologics.
An FDA analysis of drug prices from 1999 to 2004 found that the discount from generic competition was just 6% with one generic competitor, but jumps to 48% with two generic competitors, 56% with three, 61% with four and 67% with five generic producers in a market. Within 2 years of the expiration of the patent of the popular drug Zantac in 1997, generics of Zantac accounted 90% of the treatment’s total sales, and the price for patients was about 10% of its pre-generic price. European patents on biologic treatments began to expire in 2000, and in April 2006, Sandoz and Biopartners successfully received EMEA approval for the first European biogenerics, two products containing human growth hormone.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 8: Biosimilars Development in Markets
A Biosimilars bio therapeutic item is comparative (but not identical) as far as quality, wellbeing, and viability to an effectively authorized reference item. Not at all like nonspecific little particles, it’s hard to institutionalize such naturally complex items in light of convoluted assembling forms. The worldwide biosimilars advertise is developing quickly as licenses on blockbuster biologic medications terminate and other medicinal services parts centre on lessening of expenses. Biologics are among the most elevated cost medicines on the worldwide market today, which suggests the requirement for minimal effort choices. In developing markets, biosimilars officially offer more moderate costs, which are alluring, as well as crucial to economies where costly medications are not monetarily achievable
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of Bioanalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 9: Biosimilars Approval to Biogenerics in Clinical Practice
Biological medicines are much more structurally complex and extremely sensitive to manufacturing conditions and therefore more difficult to characterize and produce than small molecule drugs. Even minor changes in manufacturing may lead to significant variations of the cellular systems used for biological production, as well as to differences in the structure, stability, or other quality aspects of the end product, all of which have the potential to affect tolerability and/or efficacy and increase the risk of immune responses. Owing to these issues, specific regulatory guidance on biosimilars is continuously evolving, and there is some disagreement on which studies need to be implemented to approve a biosimilars. According to current literature, the following points on biosimilars deserve consideration: Biosimilars development is characterized by global harmonization, although several not fully answered questions remain regarding extrapolation of indications, switching or interchange ability, and tolerability; in patients with rheumatic diseases, the tolerability and efficacy of biosimilars in clinical practice remain to be established; several medical and patient associations have published position papers on biosimilars requesting that safety, efficacy, and traceability be carefully considered; long-term post marketing studies should be implemented to allow physicians to gain confidence in biosimilars.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 10: Biopharmaceutical Informatics
Biopharmaceutical informatics endeavours to use information technology, sequence-and structure-based bioinformatics analyses, molecular modelling and simulations, and statistical data analyse towards biologic drug development. Development of databases containing the experimental data on biophysical stability, safety along with molecular sequence
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 11: Consequences of Brexit on Biosimilars
With Europe that paved way to the uptake of biosimilars over a decade ago, the consequences of Brexit would be potentially harder on UK. Presently UK is no more bound to follow the guidelines of EMA. Also research grants from Innovative Medicines Initiative and Horizon 2020 would no more be available to UK. All the same, EMA has its headquarters in London, UK. The thus arising complications would definitely have certain consequences on the Biosimilars scenario in UK and EU.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 12: BCS and IVIVC Based Biowaivers
The objective of this work was to suggest the biowaivers potential of biopharmaceutical classification system which are known to increase the solubility, dissolution, oral absorption of water insoluble drugs. BiopharmaceuticsClassification System and invitro and invivo classification discusses about ADME pathways of different drugs. This also includes BCS biowaivers, In vitro diffusion cells for dissolution testing in formulation development, In vitro preclinical ADME/BCS testing. Until in vitro and in vivo correlation achieves the required degree, the biosimilars drug will not be able to meet the needs of the original drug candidate. Hence the proportion of BCS and IVIVC based biowaivers are fairly low ~0.5-1% of total pharmaceutical products.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 13: Biosimilars Market and Cost Analysis
The global biosimilars market is growing at an exponential rate. The CAGR from 2015 to 2020 is projected at over 22%. The biosimilars market is expected to be around $6.2 billion by 2020 from only $2.3 billion in 2015. By the end of this decade the biosimilars would surely occupy 27% of the total pharmaceutical market. Moreover, with the global rise in concern for more accessible-improved- cost effective healthcare, biosimilar drugs would be a more apt choice to the payers, end users, manufacturers over the costly reference biologics. Originator biologics are as costly as about $100,000 per year per patient. Biosimilars on the contrary can be offered at a 30-40% lower price than that of the reference product. However, with all the success stories and opportunities there also lies a sobering 50% failure rate in developing and obtaining license towards marketing of biosimilars. The biosimilars market is categorized into mainly four zones – North America(USA and Canada); Europe(UK, Germany, Spain, Italy, France and Rest of Europe); Aisa-Pacific( China, India, Japan, South Korea) and rest of the world ( LATAM and MENA). Key players of the biosimilars market include Amgen, Hospira, Teva, Sandoz International GmbH, Dr. Reddy’s Laboratory, Biocon, Roche, Celltrion, Catalent, Mylan and Merck. There are also certain other companies which are gaining importance in biosimilar de​velopment like LeanBio Pro-Spain, PPD-USA, SGS Life Sciences-UK, Therapeutic Proteins International-USA. The biosimilars development is mainly concentrated in the therapeutic domains of oncology, blood disorders, autoimmune disorders, endocrine disorders and infectious diseases.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 14: Challenges in Biosimilars Pharmacovigilance
This session of the Biosimilars 2018 will look into the future and FDA initiatives that have already been announced to include enhanced tracking and follow-up of post marketing surveillance issues, planned improvements in AERS, and pilots of new post market drug-monitoring strategies and ADR related issues. Biosimilar guidelines for pharmacovigilance practice and pharmacoepidemiology are the points that shall be laid emphasis in this session. U.S. average annual spending growth from 2002 to 2007 was 16% for biologics, compared with 3.7% for drugs. In same proportion pharmacovigilance for biosimilars has been comparatively more than other pharmaceutical products.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 15: Legal Issues and BPCI Act
The legal issues pertaining to the follow-on-biologics and biosimilars are one of the most aspects that requires an open discussion. Before the actual advent of biosimilars to the market legal issues have risen in numbers in their developmental stages. Renowned organizations have filed cases against each other two claim their rights and for other legal allegations related to the products. This track is dedicated to discussion of all such cases which has been argued in the court of law.
By 2002, the FDA had approved 36 new biologics, followed by 37 more in 2003, another 40 in 2004 and 39 more in 2005. By 2006, the leading category of biologic treatment, the red blood cell enhancer recombinant erythropoietin (EPO), generated $14 billion in sales revenues, or 40 percent more than the best-selling traditional pharmaceutical, Lipitor. More than 300 therapeutic antibodies currently are in clinical development and trials, compared to just 13 that already are widely available due to legal issues.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 16: Formulation Strategies for Follow-on Biologics
For this rapidly growing industry sector, little consensus or authoritative information is available yet regarding how and where biosimilar products will be produced. The future of their manufacturing is still up in the air. Much discussion among experts has focused on a dramatic up-tick in Contract Manufacturing Organizations (CMOs) involvement with process development projects and clinical-scale manufacturing for biosimilars. None have really set up commercial-scale projects (yet), however. Many such companies are publicly discussing large-scale partnerships for production in India and elsewhere in Asia. On the other hand, some biosimilars sponsors are establishing relationships with smaller biotech CMOs that have demonstrated expertise in efficiency or lower-cost business models.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 17: Current Agency Expectations for Approval for Biosimilars
Biosimilars are a relatively new subset of biopharmaceuticals, with the biotechnology industry finally maturing such that off-patent generic-type products increasingly will be entering major markets. So far, more than 20 biosimilars for a limited number of reference products have been approved in major markets, primarily the European Union. Only two products have been formally approved as biosimilars in the United States. The parent field of biopharmaceuticals itself continues to exhibit a poor supporting infrastructure of information resources. Those biopharmaceutical and biosimilar information resources that do exist generally are limited in number, diversity, and sophistication.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 18: Biosimilars Research Pipeline
Biosimilars is a biologic medical product which is copy of an original product that is manufactured by a different company. Biosimilars are officially approved "innovative" versions of original products, and can be manufactured when the original product's patent expires. Reference to the innovator product is an integral component of the approval. This session also finds place for all the biosimilars exhibitors associated with the field of biosimilars and biologics. Biosimilars innovative products are on the rise. The number of new drugs seeking approvals is growing at a compounded rate of around 5% half early. Almost 1.5 times the number of biosimilars is expected to be in the market in 2016 compared to in the last 5 years.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 19: Globalization of Biosimilars
This track discuses about the generic drugs impact on global biosimilars market , Cost and risk management, Adopting innovative mechanisms such as risk-sharing arrangement, European market for biosimilars. The global market scenario with the launch of first biosimilars in the market forecasts some radical changes. This track will look upon such key concerns which are witnessed by the global pharma market and that are coming up with the subsequent launch of the other biosimilars and biologics. Despite these emerging facilities, biotherapeuticdevelopers are most comfortable off-shoring to established markets—the US and Europe.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 20: Drug Delivery and Development
Drug Delivery Companies and Market session is beginning to change for small, medium, and large scale pharmaceutical Co, biopharmaceutical Manufacturing and Industries, generic drugs companies, contract drug delivery companies which can manifest from development to manufacturing. Addressing these instabilities is a great challenge, because of the complexity of the Clinical bio therapeutics themselves.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 21: Monoclonal Antibody Biosimilars
The first mAb biosimilars to be approved in Europe and elsewhere was the infliximab biosimilars (Inflectra and Remsima), which originator is Remicade. In October 2013, after the European Medicines Agency (EMA) gave a positive opinion for infliximab biosimilars, the European Commission granted a marketing authorization (MA) for these two biosimilars in the various therapeutic indications that are authorized for Remicade (i.e. indications in gastroenterology, rheumatology, and dermatology). As for previously approved biosimilars, the two aforementioned European institutions did not make a stand on the issues of substitution, and with good reason, as answering these questions does not fall within their areas of expertise and is the sole responsibility of EU Member States
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Track 22: USFDA Approved Biosimilars
In the United States, the Food and Drug Administration (FDA) held that new legislation was required to enable them to approve Biosimilars to those biologics originally approved through the PHS Act pathway. Since 2004 the FDA has held a series of public meetings on Biosimilars. The FDA has previously approved biologic products using comparability, for example, Omnitrope in May 2006, but this like Enoxaparin was also to a reference product, Genotropin originally approved as a biologic drug under the FD&C Act.
Related Conferences: Biosimilars Conferences | Biosimilars Events | Biosimilars Workshops | Biosimilars Symposiums | Biosimilars Meetings | Euro Biosimilars Conferences | Biosimilars 2019 | Generic Biosimilars Conferences | World Biosimilars Congress | Biosimilars Europe Congress | Biosimilars Congress | Biosimilars and Biologics | Biosimilars VS Generics | Biologics Conferences | Pharmaceutical Conferences
19th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry, February 25-27, 2019 at Berlin, Germany; 20th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, March 18-20, 2019 at Edinburgh, Scotland; 10th Global Organic & Inorganic Chemistry Conference, March 21-22, 2019 at Rome, Italy; 11th International Conference and Exhibition on Advances in Chromatography & HPLC Techniques, April 22-24, 2019 at Dublin, Ireland; 7th International Conference and Exhibition on Materials Science and Chemistry, May 20-22, 2019 at Zurich, Switzerland; 21st Annual European Pharma Congress, May 20-21, 2019 at Zurich, Switzerland; 5th International Conference on Electrochemistry, May 27-28, 2019 at Barcelona, Spain; 6th World Congress and Exhibition on Antibiotics and Antibiotic Resistance, June 03-04, 2019 at London, UK; 22nd International Conference and Exhibition on Pharmaceutical Formulations, June 03-04, 2019 at London, UK; 11th World Congress on Medicinal Chemistry and Drug Design, June 10-11, 2019 at Edinburgh, Scotland; 8th World Congress on Mass Spectrometry, June 10-11, 2019 at Edinburgh, Scotland; 6th International Conference and Exhibition on Natural Products Chemistry and Medicinal Plants Research, June 24-25, 2019 at Vienna, Austria; 23rd International Conference and Exhibition on Pharmacovigilance & Drug Safety, July 4-5, 2019 at Valencia, Spain; 24th World Congress on Pharmacology, August 19-20, 2019 at Vienna, Austria; 5th International Conference on Advanced Clinical Research and Clinical Trials, September 16-17, 2019 at Rome, Italy; 9th International Conference and Expo on Separation Techniques, October 14-15, 2019 at London, UK
Related Societies:
Societies in USA: Canadian Society for Pharmaceutical Sciences (CSPS), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT), American Society of Health-System Pharmacists (ASHYP). Canadian Society of Intestinal research (CSIR)
Societies in Europe: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS), Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI). A Division of the Association for Accessibility medicines (DAAM)
Societies in Asia and Pacific: The Pharmaceutical Society of Australia (PSA), Austrian Pharmaceutical Society (APS), Korean Research-based Pharmaceutical Industry Association (KRPIA), Kuwait Pharmaceutical Association (KPA).
Related Journals:
Journal of Bioanalysis & Biomedicine, Enliven: Biosimilars and Bioavailability, Journal of Bioequivalence & Bioavailability, MOJ Bioequivalence & Bioavailability, Bioequivalence Study Journal, Chemical Speciation & Bioavailability, International Journal of BioAnalytical Methods & BioEquivalence Studies, Asian Journal of Pharmaceutical & Biological Sciences, Bioanalytical Methods & Bioequivalence Studies, Pharmaceutical Regulatory Affairs: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Generics and Biosimilars Initiative Journal, The pharmaceutical Journals, The American Pharmacists Association, Applied Basic Medical Research, Inventi Impact – Biosimilars, journal of Oncology Practice
Biosimilars: Global Markets
As per market researchers the global "Biosimilars Market by Product (Recombinant Non-Glycosylated Proteins (Insulin, rHGH, Interferon), Glycosylated (mAb, EPO), Peptides (Glucagon, Calcitonin)), Manufacturing Type (In-house, Contract), Disease (Oncology, Autoimmune) - Global Forecast to 2023", The biosimilars market is expected to reach USD 23.63 Billion by 2023 from USD 5.95 Billion in 2018, at a CAGR of 31.7%. Granulocyte colony-stimulating factors (G-CSFs) reached $379.3 million in 2013. This segment is expected to increase from $453.6 million in 2014 to $1.1 billion by 2019, a CAGR of 20.2% from 2014 to 2019.
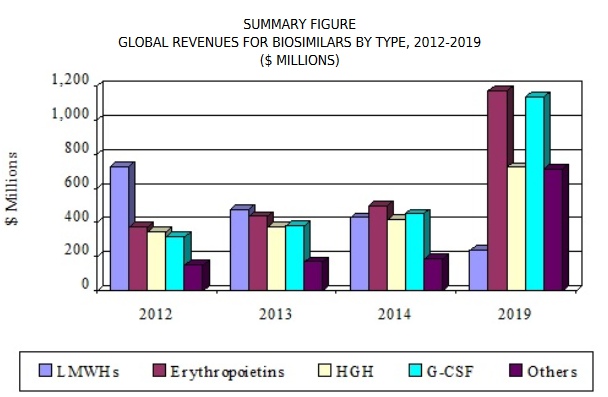
Source: BCC Research
Importance & Scope:
The European-based pharmaceutical industry makes a major contribution to the Europe, not just in financial terms but also in terms of high-trait employment. Globally Pharma Market ranges from $870-$900 billion and in Europe $260-$280 billion.
A global biosimilars strategy:
Developed markets: Developed markets, with the exception of the United States, represent the greatest biosimilars presence today. Most biosimilars manufacturers have been and remain focused on the developed markets – whether it is for their historic and current opportunities (EU) or for their future market potential (United States, Japan). Dedicated regulatory pathways set the foundation for stringent, abbreviated approval processes which, in turn, have fed investor enthusiasm. Biosimilars adoption in developed markets has been primarily payer-driven, especially in European markets, given payers’ urgent, unmet need to contain public health care expenditures. Further market uptake has been slowed by prescribers’ skepticism and low patient awareness. Still, developed markets continue to have the highest number of biosimilars molecules in development – estimated at 29 in Europe, 19 in the United States and seven in Japan.
Emerging markets: In today’s emerging markets, biosimilars are still nascent, with little to no presence. However, in contrasting emerging markets with developed markets, the limited patient access to affordable biologics and the openness of physicians to low-cost therapies may offer potentially significant opportunities. Today, emerging markets represent a snippet of total world biologic sales in value, less than seven to eight percent (versus 48.6 percent in the United States).ix Treatment rates for flagship biologics are still low compared to developed markets, despite existing demand. For example, the treatment rate of MabThera® in Brazil is three times lower than in the UK and six times lower than in the US.x Additionally, a recent Kantor Health Survey found that 20 percent of emerging market autoimmune patients use a biologic, with the distribution of biologics varying from 29 percent in China to 12 percent in Russia and a mere 6 percent in Brazil.xi This may indicate the presence of large pockets of non-consumption, especially within the growing middle class.
Biosimilars Market Projected To Reach $41.7 Billion By 2024
Biosimilars Market, by Product (Revenue, USD Million, 2017 - 2024)
-
Recombinant Non-Glycosylated Proteins
- Insulin
- Human Growth Hormones
- Granulocyte Colony-stimulating Factor (G-CSF)
- Interferons
-
Recombinant Glycosylated Proteins
- Erythropoietin
- Monoclonal Antibodies
- Follitropin
Biosimilars Market, by Application (Revenue, USD Million, 2017 - 2024)
- Oncology
- Blood Disorders
- Growth Hormone Deficiency
- Chronic and Autoimmune Disorders
- Others
Biosimilars Market, by Region (Revenue, USD Million, 2017 - 2024)
-
North America
- U.S.
- Canada
-
Europe
- UK
- Germany
-
Asia Pacific
- China
- India
-
Rest of the World
- Brazil
- Russia
Analysis of selected countries:
United States
- FDA approval of the first biosimilar in March 2015 with Sandoz’s Zarxio (filgrastim)
- About 19 pipeline biosimilar molecules in development
- Represents about 50% of the global biologics market value and generates about 50% of the sales value growth
- Pending legislative decisions on data exclusivity period, naming conventions and interchangeability likely to have important implications.
Biosimilars approved in the US
In the US, a legal framework for approving biosimilars was established in 2009, via the Biologics Price Competition and Innovation Act of 2009 (BPCI Act).
The BPCI Act is part of the healthcare reform legislation, which was signed into law on 23 March 2010 by President Barack Obama. The BPCI Act establishes an abbreviated approval pathway for biological products that are demonstrated to be ‘highly similar’ (biosimilar) to, or ‘interchangeable’ with, a US Food and Drug Administration (FDA)-licensed biological product.
FDA is still in the process of developing guidelines regarding these types of products and has issued several guidance documents on the subject.
Zarxio (filgrastim-sndz) was the first product approved in the US as a biosimilar in 2015. To date, FDA has approved four biosimilars within the product classes of anti-tumour necrosis factor-alpha (TNF-α) and granulocyte colony-stimulating factor, and a follow-on biological in the product class of insulin for use in the US.
FDA approved Biosimilars and follow-on Biologicals
|
Product name |
Active substance |
Therapeutic area |
Authorization date |
Manufacturer/ Company name |
|
Amjevita (adalimumab-atto) |
adalimumab |
Ankylosing spondylitis |
23 Sep 2016 |
Amgen |
|
Basaglar# |
insulin glargine |
Diabetes |
16 Dec 2015 |
Eli Lilly/Boehringer Ingelheim |
|
Epoetin Hospira |
epoetin alfa |
Anaemia (chronic kidney disease, Zidovudine, chemotherapy) |
Recommended for approval by FDA’s Oncologic Drugs Advisory Committee (ODAC) on 25 May 2017 |
Pfizer (Hospira) |
|
Erelzi (etanercept-szzs) |
etanercept |
Axial spondyloarthritis |
30 Aug 2016 |
Sandoz |
|
Inflectra |
infliximab |
Ankylosing spondylitis |
5 Apr 2016 |
Pfizer (Hospira) |
|
Renflexis (infliximab-abda) |
infliximab |
Ankylosing spondylitis |
21 Apr 2017 |
Samsung Bioepis |
|
Zarxio |
filgrastim |
Autologous peripheral blood progenitor cell collection and therapy |
6 Mar 2015 |
Sandoz |
|
*Data collected on 30 September 2016, updated 26 June 2017 Source: US FDA |
||||
EU5
- Most mature biosimilar market representing 80% of global biosimilar spending
- Performance to date viewed as “disappointing” by select manufacturers
- Nineteen biosimilar products authorized in four molecule classes: human growth hormone, erythropoietin, G-CSF and tumour necrosis factor (TNF)-inhibitor
- About 29 pipeline biosimilars molecules in development.
- World-class dedicated pathway leaving questions of substitutability at the pharmacy level to member states
- Payer-driven uptake
- Challenged by continued pressure from strict regulatory decisions, lingering fear from prescribers around biosimilars’ “similarity”, safety and efficacy, debates on automatic substitution and INN prescription.
Biosimilars approved in Europe
In the European Union (EU), a legal framework for approving biosimilars was established in 2003. This framework means that biosimilars can only be approved centrally via the European Medicines Agency (EMA) and not nationally.
EMA first developed guidelines for the approval of biosimilars via an abbreviated registration process during 2005 to 2006, and since then EMA has developed many general and specific guidelines for biosimilars .
Omnitrope (somatropin) was the first product approved in the EU as a biosimilar in 2006. To date, EMA has approved 38 biosimilars within the product classes of human growth hormone, granulocyte colony-stimulating factor, erythropoesis stimulating agent, insulin, follicle-stimulating hormone (FSH), parathyroid hormone and tumour necrosis factor (TNF)-inhibitor, for use in the EU. Three biosimilar approvals have been withdrawn; two for filgrastim biosimilars: Filgrastim ratiopharm in April 2011 and Biograstim in December 2016, and one for a somatropin biosimilar (Valtropin) in May 2012. This leaves a total of 35 biosimilars approved for use in Europe.
EMA approved Biosimilars:
|
Product name |
Active substance |
Therapeutic area |
Authorization date |
Manufacturer/Company name |
|
Abasaglar (previously Abasria) |
insulin |
Diabetes |
9 Sep 2014 |
Eli Lilly/Boehringer |
|
Abseamed |
epoetin alfa |
Anaemia |
28 Aug 2007 |
Medice Arzneimittel Pütter |
|
Accofil |
filgrastim |
Neutropenia |
18 Sep 2014 |
Accord Healthcare |
|
Amgevita |
adalimumab |
Crohn’s disease |
22 Mar 2017 |
Amgen |
|
Benepali |
etanercept |
Axial spondyloarthritis |
14 Jan 2016 |
Samsung Bioepis |
|
Bemfola |
follitropin alfa |
Anovulation (IVF) |
24 March 2014 |
Finox Biotech |
|
Binocrit |
epoetin alfa |
Anaemia |
28 Aug 2007 |
Sandoz |
|
Biograstim |
filgrastim |
Cancer |
15 Sep 2008 |
CT Arzneimittel |
|
Blitzima |
rituximab |
Non-Hodgkin lymphoma |
CHMP positive opinion on 18 May 2017 |
Celltrion |
|
Epoetin alfa Hexal |
epoetin alfa |
Anaemia |
28 Aug 2007 |
Hexal |
|
Erelzi |
etanercept |
Ankylosing spondylitis |
CHMP positive opinion on 21 April 2017 |
Sandoz |
|
Filgrastim Hexal |
filgrastim |
Cancer |
6 Feb 2009 |
Hexal |
|
Filgrastim ratiopharm |
filgrastim |
Cancer |
15 Sep 2008 |
Ratiopharm |
|
Flixabi |
infliximab |
Ankylosing spondylitis |
26 May 2016 |
Samsung Bioepis |
|
Grastofil |
filgrastim |
Neutropenia |
18 Oct 2013 |
Apotex |
|
Inflectra |
infliximab |
Ankylosing spondylitis |
10 Sep 2013 |
Hospira |
|
Inhixa |
enoxaparin sodium |
Venous thromboembolism |
15 Sep 2016 |
Techdow Europe |
|
Insulin lispro Sanofi |
Insulin lispro |
Diabetes mellitus |
CHMP positive opinion on 18 May 2017 |
Sanofi-Aventis |
|
Lusduna |
insulin glargine |
Diabetes |
4 Jan 2017 |
Merck (MSD) |
|
Movymia |
teriparatide |
Osteoporosis |
11 Jan 2017 |
STADA Arzneimittel |
|
Nivestim |
filgrastim |
Cancer |
8 Jun 2010 |
Hospira |
|
Omnitrope |
somatropin |
Pituitary dwarfism |
12 Apr 2006 |
Sandoz |
|
Ovaleap |
follitropin alfa |
Anovulation (IVF) |
27 Sep 2013 |
Teva Pharma |
|
Ratiograstim |
filgrastim |
Cancer |
15 Sep 2008 |
Ratiopharm |
|
Remsima |
infliximab |
Ankylosing spondylitis |
10 Sep 2013 |
Celltrion |
|
Retacrit |
epoetin zeta |
Anaemia |
18 Dec 2007 |
Hospira |
|
Ritemvia |
rituximab |
Wegener granulomatosis |
CHMP positive opinion on 18 May 2017 |
Celltrion |
|
Rixathon |
rituximab |
Chronic B-cell lymphocytic leukaemia |
CHMP positive opinion on 21 April 2017 |
Sandoz |
|
Riximyo |
rituximab |
Chronic B-cell lymphocytic leukaemia |
CHMP positive opinion on 21 April 2017 |
Sandoz |
|
Silapo |
epoetin zeta |
Anaemia |
18 Dec 2007 |
STADA R & D |
|
Solymbic |
adalimumab |
Ankylosing spondylitis |
22 Mar 2017 |
Amgen |
|
Terrosa |
teriparatide |
Osteoporosis |
4 Jan 2017 |
Gedeon Richter |
|
Tevagrastim |
filgrastim |
Cancer |
15 Sep 2008 |
Teva Generics |
|
Thorinane |
enoxaparin sodium |
Venous thromboembolism |
15 Sep 2016 |
Pharmathen |
|
Truxima |
rituximab |
Chronic lymphocytic leukaemia |
17 Feb 2017 |
Celltrion |
|
Tuxella |
rituximab |
Wegener granulomatosis |
CHMP positive opinion on 18 May 2017 |
Celltrion |
|
Valtropin |
somatropin |
Pituitary dwarfism |
24 Apr 2006 |
BioPartners |
|
Zarzio |
filgrastim |
Cancer |
6 Feb 2009 |
Sandoz |
|
*Data collected on 30 September 2016, updated 26 June 2017 Source: EMA |
||||
Japan
- Limited maturity of the biosimilar market
- Dedicated regulatory pathway
- About seven pipeline biosimilar molecules in development
- Growth potential considered limited today based on the reluctance from both prescribers and patients as well as the general mistrust toward “generic makers”
- A push from payers, which has yet to be seen, may help open up the market.
Brazil
- Led the way with the development of biosimilars regulations in Latin America and released biosimilars guidance in 2010
- Reducing the reliance on imported (and high-cost) medicines through policies that favor the expansion of the domestic pharmaceutical industry and public-private partnerships to expand access to drugs
- International companies have entered the market through partnerships and acquisitions (e.g., Pfizer’s 40% stake in Teuto, Sanofi’s acquisition of Medley and Merck’s joint venture with Supera, co-owned by Cristalia and Eurofarma)
- The regulatory environment and interest of domestic and international manufacturers are major drivers in expanding the biosimilars market.
- Approximately five biosimilar molecules in the development pipeline.
- Seventy-five percent of physicians surveyed in Brazil considered rituximab difficult to access due to high costs and 77% said they would increase prescription of rituximab if a cheaper alternative were available.
Russia
- Aims to boost its domestic pharmaceutical market and increase the market share of domestic players from 20% in 2012 to 50% by 2020
- The strong preference for local manufacturers will require international companies to engage in cooperative partnerships with Russian companies
- Indicative of the burgeoning domestic industry, a rituximab biosimilar, developed by Russian company Biocad, was the first mAb biosimilar approved in Russia in April 2014
- About eight biosimilar molecules in the development pipeline.
India
- Biosimilar guidelines established in 2012
- 80% of pharmaceutical spend is out of pocket
- Indian companies have extensive experience with generics and have made in-roads in other countries as well through exports
- Indian companies grapple with the image of manufacturing as unsafe with poor quality drugs
- Partnerships between global pharmaceutical companies and domestic companies are helping to improve the quality of biosimilars marketed in India
- Approximately 19 biosimilars in the development pipeline; large proliferation of non-original biologics
- Large middle class with growing disposable income who prefer brand name products, so there is a good opportunity for branded Biosimilars.
Approximately 70% of the country’s population is considered rural and will focus on the cost of therapy – a 20-30% discount on originator biologics may not be sufficient.
- Issued draft biosimilars guidelines in 2014; once a clear regulatory pathway for biosimilar approval is established, the market will be very attractive – not only due to the volume potential but also the growing ability to pay
- Similar to the tight controls requiring international companies to create partnerships or use domestic pharmaceutical distributors, the successful manufacturing and marketing of biosimilars will also require partnerships with domestic companies
- Lack of physician trust and enthusiasm for non-branded drugs exacerbated by unsafe and counterfeit drugs
- Sophisticated market; generics make up more than 50% of the market
- Biosimilars guidelines were established in 2010
- There is a financial pressure on the system overall, and great pressure to utilize generics including biosimilars
- Several Indian companies have entered the South African market and are key to keeping drug costs low
- There is a cost containment focus from the government and payer side and a quality focus from the physician and patient side
- Companies will have to bring in a cost structure that is lower than what currently exists along with the highest quality and safety profiles of their biosimilars
- Established, government-incentivized market for biosimilars
- Demand spurred by high out-of-pocket health care spending (estimated at +90%)
- Significant presence of non-original biologicals known as “biolimbos” who have not undergone marketing authorization review consistent with globally accepted standards.
- Biosimilars development led locally by Probiomed which won six biosimilars approvals, including a version of Rituxan®
- Most mature biosimilar “development” market
- Enabled by unprecedented support from the South Korean government: 35% of the national medical R&D budget was invested into biosimilars development in 2012xvi
- Government-set goal for domestic biopharmaceutical companies to win 22% of the global biosimilars market by 2020
- Twelve biosimilars have been approved and another 36 biosimilars are in the pipelinexvii
- Leading the race in the high-risk and complex development of monoclonal antibody (mAb) biosimilars with 17 mAbs in the pipeline
A recent study revealed that a third of anti-malaria drugs sold were found to be counterfeit, and 100,000 deaths per year in Africa were linked to counterfeit drugs. Physicians and patients in emerging markets are particularly wary of non-brand name drugs. 75% of emerging markets pharmaceutical growth is expected to come from branded generics. According to a 2013 Roper Report, 79 percent of consumers in developing Asian markets and 61 percent of consumers in Latin American markets only buy products and services from a trusted brand.
Main factors that support a sustainable european biosimilar drugs market:
A study conducted by the consultancy company GFK on behalf of the European Biosimilars Group It analysed the main factors that support a sustainable European biosimilar drugs market. The study revealed that biosimilar medicines provide a major opportunity with considerable benefits, since they lead to cost savings throughout Europe, contribute to the sustainability of National Healthcare Systems and improve patient access to innovative treatments. However, in order to achieve these benefits in the long term, the biosimilar medicines market must continue to be sustainable.
The study concluded that the main factor influencing the sustainability of the biosimilar medicines market and enabling biosimilar drug implementation is that this market must be attractive and deliver continuing benefits, in both the short and long term, to the four key stakeholder groups: physicians, payers, patients and industry. However, the study emphasises that the “attractiveness” and “benefit” concepts are different in each of these groups. In this way, for physicians it involves having more opportunities to treat more patients with appropriate therapies. For payers, it entails cost savings and the financial sustainability of healthcare systems. For patients, it means having improved access to medicines. Finally, for the industry, it involves a reasonable return on investment with the continued attractiveness of R&D investment in new medicines development.
On the other hand, other factors that strongly influence the development and implementation of biosimilars include the prescribing physician’s trust, the complexity of clinical development and the uncertain regulatory framework. In order to increase the prescribing physicians’ trust, they must have a good understanding of biosimilars. In fact, as we already stated in a previous post, the consultancy company PwC (PricewaterhouseCoopers) revealed that only 17% of survey respondents chose the correct biosimilar definition from a list provided (PwC Health Research Institute, Top Issues Consumer Survey, 2015). Therefore, a series of training programs have been established providing clear information about them, including courses, discussion forums, conferences and newsletters. The complexity of clinical development is another important factor because it has a direct impact on obtaining marketing authorisation approval from regulatory authorities, such as the Food and Drug Administration (FDA) in the Unites States, and the European Medicines Agency (EMA) in Europe. The more complex a drug’s clinical development is, the more time is required to obtain the marketing authorisation and the longer it takes to implement it. Finally, the legal framework regulating the development, approval, marketing and implementation of biosimilars, depending on the country, is quite uncertain and vague. However, it is true that Europe has been pioneering in the establishment of a legal framework and guidelines for the approval of biosimilars. In fact, the Spanish Ministry of Health, Social Services and Equality published the Price Referencing Order (SSI/1305/2016) in Spain’s Official State Gazette (BOE) which marks the introduction of biosimilars into the health system.
Critical Analysis:
The capital-intensive nature of the biosimilar business and long gestation periods between initial investment and commercialization require biosimilar players to take a higher level of risk than their counterparts in the smallmolecule generics business, and this can be a significant barrier to entry for many start-ups.
Major Biosimilars Associations in Europe
Conference Highlights
- Current Challenges in Developing Biosimilars
- Chemical and Analytical Strategies for Biosimilars
- Intellectual Property Rights
- Biological Medicine
- Emerging Biosimilars in Therapeutics
- Innovative Clinical Approach in Biosimilars
- Regulatory Updates on Biosimilars
- Biosimilars Development in Markets
- Biosimilars Approval to Biogenerics in Clinical Practice
- Biopharmaceutical Informatics
- Consequences of Brexit on Biosimilars
- BCS and IVIVC Based Biowaivers
- Biosimilars Market and Cost Analysis
- Challenges in Biosimilars Pharmacovigilance
- Legal Issues and BPCI Act
- Formulation Strategies for Follow-on Biologics
- Current Agency Expectations for Approval for Biosimilars
- Biosimilars Research Pipeline
- Globalization of Biosimilars
- Drug Delivery and Development
- Monoclonal Antibody Biosimilars
- USFDA Approved Biosimilars
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | April 15-16, 2019 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | ||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Bioanalysis & Biomedicine
- Journal of Bioequivalence & Bioavailability
- Journal of Pharmaceutical Sciences & Emerging Drugs
Abstracts will be provided with Digital Object Identifier by